Groundwater is an essential natural resource. Ninety percent of rural residents and 50 percent of the total population in the United States depend on groundwater as a source of drinking water. Concern about its quality and potential contamination has made groundwater protection a national issue.
There are several reasons for the widespread dependence on groundwater. In its natural state, groundwater is usually of excellent quality and can be used without costly treatment or purification. It can be inexpensively tapped adjacent to the point of use, thereby saving the cost of transporting water long distances. For rural residents relying on individual wells and for public water supplies in some communities, groundwater is often the only available water supply.
The Nature of Groundwater
Consumption of groundwater is increasing at twice the rate for surface water. This trend is expected to continue as the demand for water increases in the future. Protecting the quality of existing and potential future groundwater supplies is therefore an issue of utmost importance. The soil plays a significant role in any groundwater protection strategy. This fact sheet describes how good soil management can help in protecting our groundwater resources.
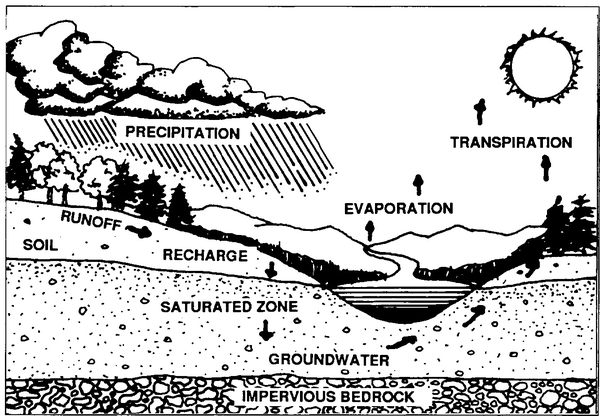
Understanding groundwater in its environmental context is vital to appreciating its value. Water moves from the atmosphere to the earth's surface, into the ground, and eventually back into the atmosphere. These interrelationships, including the important role played by the soil, are illustrated in Figure 1.
The geologic formation through which groundwater moves is called an aquifer. It can be a layer of sand, gravel, or other soil materials, or a section of bedrock with fractures through which water can flow. Groundwater does not consist of large underground lakes or streams. Rather, it occupies spaces within rock fractures or between particles of sand, gravel, silt, or clay. Furthermore, groundwater does not move rapidly in the aquifer. It may move only a few feet per month or even per year, whereas surface streams flow several feet per second.
The Role of the Soil
Both the quantity and quality of groundwater depend on the water that moves down through the soil to the saturated zone. This percolating water, called recharge, passes through the root zone and unsaturated zone until it reaches the water table. The soil is a controlling factor in the recharge process because it may hold the water in soil pores, release it to plant roots or the atmosphere, or allow it to pass through to the underlying materials. Efforts to protect groundwater focus primarily on the recharge process because it controls both the quantity and quality of water reaching the saturated zone.
Groundwater is diffuse, vulnerable, and potentially affected by almost all types of land uses and activities. It is one part of a sensitive, highly interdependent system. It becomes contaminated when recharge water carries pollutants down into the water table. For example, chemicals on the soil surface or incorporated into the soil can become groundwater contaminants if they are carried by the recharge water.
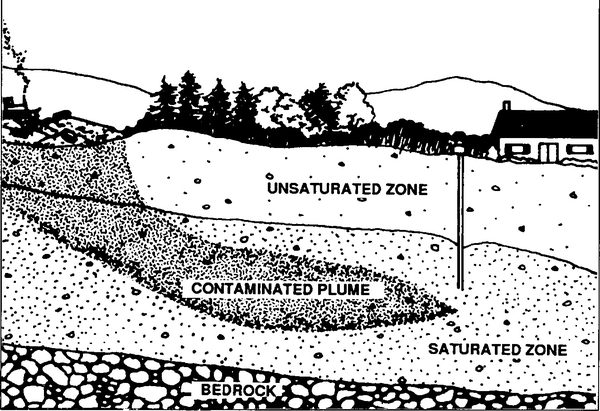
Once the recharge water reaches the aquifer, it travels in a more horizontal direction in response to pressure gradients within the aquifer. Chemicals in the recharge water move with the groundwater, forming a region of contaminated water called a plume (Figure 2). This plume may flow with the groundwater until it eventually resurfaces, thus contaminating springs, wells, streams, wetlands, or other bodies of surface water.
The soil has the ability to filter potential pollutants and prevent them from reaching groundwater. However, soils vary tremendously in their adsorption or filtering capacity. As a result, under some conditions, soluble materials take months or years to move from the land surface to an aquifer. Under other conditions, they can flow almost directly into the groundwater. An understanding of soil characteristics is essential in assessing the potential for groundwater contamination in a given situation.
Soil Characteristics
Depth. Depth to groundwater is important primarily because it deter mines the volume of soil through which a contaminant must travel before reaching an aquifer. It also determines the amount of time that a contaminant is in contact with the soil. Where the soil is fairly deep, the processes of filtration, sorption, biodegradation, and volatilization operate effectively. Conversely, shallow soils (less than 36 inches deep) can absorb only a limited amount of pollutants. The pollution potential increases where the soils are thin and the underlying bedrock is permeable, or where the water table is near the surface.
Texture. The relative proportion of sand, silt, and clay in a soil deter mines its texture - that is, its fineness or coarseness. Texture influences the movement of water through a soil by its effect on size of pores and total surface area. Sandy soils have large pores and low surface areas, allowing water to drain rapidly. Rapid leaching of some potential groundwater pollutants may result. The sandhills region is characterized by soils of sandy texture.
Clay particles provide a vast sur face area on which sorption can take place. As a result, leaching (percolating through the soil) is less likely in clay soils. Most of the Piedmont region is characterized by soils with clay subsoils.
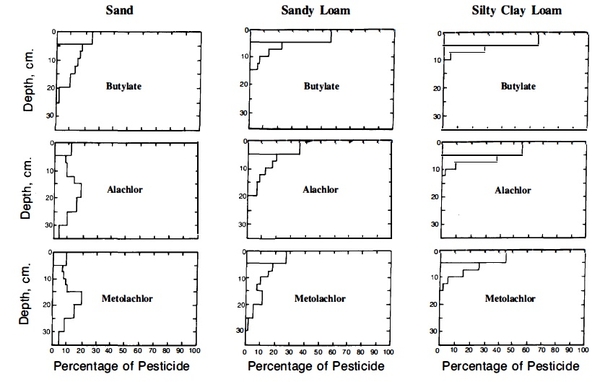
The influence of soil texture on the leaching of three popular herbicides is shown in Figure 3. Downward movement of the herbicides is greatest in the sand and least in the silty clay loam. The increased adsorption near the surface, especially in the finer-textured soils, is caused mainly by the presence of organic matter.
Organic Matter Content. Well humified organic matter has a very large adsorptive capacity for both organic and inorganic compounds, including most pollutants. For most soils, organic matter is concentrated in the topsoil. Maintaining an active organic component in the topsoil through good soil and crop management enhances the soil's capacity to serve as a filter.
Another beneficial effect of organic matter is its improvement of soil structure. Structure refers to the massing of individual soil particles into larger aggregates. Organic matter tends to promote desirable aggregation and thus increases the sorptive capacity of the soil. Soil structure can be affected by land use and management. For example, tilling the soil under the proper moisture condition and incorporating crop residues enhance soil structure.
Characteristics of Potential Pollutants
The potential for groundwater pollution is influenced not only by the soil but also by characteristics of the various contaminants. This section describes how the specific characteristics of nitrate, pesticides, and biological contaminants affect their potential for contaminating ground water.
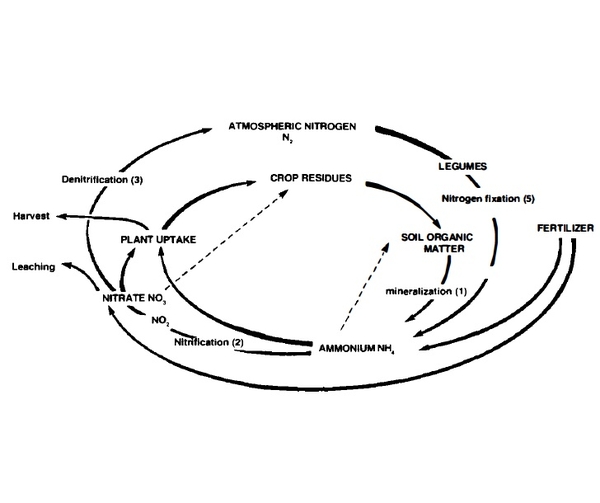
Nitrogen. Nitrogen (N) is one of the most difficult elements to trace through the environment because of the many chemical paths it can follow, as illustrated by the nitrogen cycle in Figure 4. The diagram shows why nitrogen is difficult to manage completely and efficiently.
The nitrification process (path 2 in Figure 4) produces nitrate (N03), which may have a significant effect on groundwater. Nitrate is water soluble and highly mobile and is thus subject to leaching. Nitrification is also a key to high crop yields because crops need a certain level of nitrate in solution and grow better when a significant portion of their nitrogen is supplied as nitrate. Be cause plants remove nitrate from the root zone, higher nitrate concentrations are usually found in the soil away from the roots. Water moving through the soil during the summer or after the growing season can carry this "excess" nitrate to groundwater.
Pesticides. Between 1950 and 1980, production of synthetic organic pesticides more than tripled in the United States from about 400 million pounds in 1950 to over 1.4 billion pounds in 1980. Over 80 pesticides are estimated to have the potential for moving into groundwater.
Some pesticides are volatile, that is, they evaporate into the atmosphere in the same manner that water evaporates. Volatile compounds may become groundwater contaminants if they are applied below the soil surface. Pesticides that enter the soil are degraded by sunlight, by microorganisms in the soil, and by chemical and physical processes. The longer the compound withstands degradation, the longer it is subject to the forces of leaching and the more likely it is to reach groundwater.
The likelihood of a pesticide moving downward depends largely on its ability to dissolve in water, or its solubility. If a pesticide is highly soluble, it is more likely to reach the groundwater. On the other hand, many pesticides, even some that are soluble, are likely to stick to soil particles by adsorption. Thus, if the probability of adsorption is high, less of the chemical will leach.
Greatest care needs to be taken with those pesticides that are highly soluble, that do not adsorb strongly to soil particles, and that persist for a long time in the soil. The Environmental Protection Agency has established a list of such pesticides, called suspected leachers, for which extra precautions should be used to prevent contamination of groundwater supplies. Some of these suspected leachers are listed in Table 1. Those that have been detected in groundwater are marked with an asterisk.
| Chemical Name: |
|---|
| Acifluorfen |
| Alachlor* |
| Aldicarb* |
| Ametryn |
| Atrazine* |
| Bromacil* |
| Carbofuran* |
| Chloramben |
| Cyanazine* |
| Dalapon |
| DBCP* |
| Dacthal/DCPA* |
| 1,2-dichloropropane* |
| Dinoseb* |
| Disulfoton |
| EDB* |
| Fenamiphos (Nemacur) |
| Fluometuron |
| Hexazinone |
| Methomyl |
| Metolachlor* |
| Metribuzin* |
| Oxamyl* |
| Pichloram |
| Prometone |
| Pronamide |
| Propazine |
| Simazine* |
| Tebuthiuron |
| Terbacil |
* Chemicals that have been detected in groundwater.
Management Practices
A wide variety of management practices can minimize the threat of groundwater contamination from agricultural activities. Some proven practices are broadly applicable to most sites and farming situations in North Carolina, whereas others are still being tested.
Nitrogen Management on Cropland
The basic soil management principle for minimizing nitrate contamination of groundwater is to minimize the amount of available nitrogen in the root zone except when the crop is actively assimilating it. Although this concept is simple, it is difficult to apply. It requires attempting to match the available nitrogen with crop needs and managing the system so that the nitrogen is there when needed. However, even with the best management some nitrate will not be used by crops and will thus be available for leaching.
Growers can take several steps to help match fertilizer rates to crop needs. One is to take into account all nitrogen sources when determining the amount of nitrogen to apply to cropland. Organic nitrogen sources are often overlooked. For example, several studies have shown that farmers often ignore nitrogen contributions from legumes and organic wastes when they calculate fertilizer needs. As a result, there is more nitrogen than is needed by the crops, and potential for nitrate to leach into groundwater is increased. Timing of nitrogen applications is important because the greatest amount of nitrate loss occurs between the time the fertilizer is applied and the time the nitrogen is taken up by the crop. However, applying fertilizers during the period of maximum crop nitrogen demand is often not practical. Some measures that can be taken include delaying the application until after planting (sidedressing) to improve plant nitrogen-use efficiency compared to preplant applications, and splitting the nitrogen between two or more applications during the growing season. These techniques are particularly effective with crops like com and wheat in avoiding nitrogen losses on sandy soils.
Land application of organic wastes, including farm manures, can cause nitrate to accumulate in the soil and lead to groundwater pollution. Household wells are often located close to animal holding areas, presenting the possibility that drinking water will be contaminated by nitrate and coliform bacteria. Despite very high nitrogen loading rates, feedlots and barnyards occupy a small portion of the landscape, and they can be managed to minimize pollution problems.
The current strategy in North Carolina is to apply wastes at a rate that will match the crops' nitrogen needs. This approach minimizes the leaching of nitrate to groundwater. However, the amount of manure that can be applied to a given area over the long term may need to be determined by the accumulation of phosphorus rather than nitrogen. Phosphorus is not a groundwater concern but its presence in large amounts in the top few inches of soil poses a major concern for surface water contamination.
Pesticide Management
Careful on-farm pesticide management can significantly reduce the potential for pesticide contamination of groundwater. Good management practices include selecting the proper kind and amount of pesticide, applying it properly, and storing it safely.
On-farm handling, which includes transportation, mixing, loading, and storage, must be managed carefully to protect groundwater. Accidental spills during these operations can cause significant amounts of a pesticide to move into the soil. Even a number of small spills can lead to an accumulation of pesticides in the soil, increasing the potential for leaching to groundwater.
Cultural Practices
Tillage operations such as traditional moldboard plowing, various forms of conservation tillage, and cultivation for weed control can affect the potential for groundwater contamination. Tillage practices affect soil porosity and surface rough ness, which in turn affect rates of runoff, evaporation, and infiltration. As a general rule, any tillage practice that reduces leaching will help reduce the risk of groundwater contamination.
Selecting and adopting correct tillage practices is important to both groundwater and surface water quality. The aim is to reduce runoff while simultaneously maximizing the water-holding capacity of the soil to reduce leaching. Reducing leaching may lead to an increase in runoff when rainfall is high and the soil is shallow or has a low water infiltration rate.
Planting strategies can also influence the types and number of pests and pest control options. For example, planting crops in narrow rows can enhance weed control. The denser canopy protects soil from exposure to rain and sup presses growth of late-germinating weeds through shading and competition. Thus, less herbicide may be needed to control weeds.
Conclusion
Agricultural activities can lead to contamination of groundwater, mainly with nitrate and pesticides. The potential for groundwater contamination at a given location depends on site characteristics, characteristics of individual pollutants, and agronomic and management factors. Many best management practices are available to protect groundwater. The key is to identify and select those that are technically efficient and that also reduce the risk of groundwater contamination.
References and Suggested Readings
Hornsby, A.G. Ground Water: The Hidden Resource. 1986. Soil Science Fact Sheet SU8. Florida Cooperative Extension Service.
Keeney, D.R., B. Webendorfer, G. Jackson, T. C. Daniel, and B. Shaw. 1986. Nitrate in Wisconsin Groundwater-Sources and Concerns. Extension Bulletin G 3054. University of Wisconsin, Madison.
Porter, K. S. and M. W. Stimmann. 1988. Protecting Groundwater: A Guide for the Pesticide User. Washington D.C.: U.S. Department of Agriculture and U.S. Environmental Protection Agency.
Spillner, C. J., V. M. Thomas, and D. G. Takahashi. 1983. A comparative study of the relationships be tween the mobility of alachlor, butylate, and me tolachlor in soil and their physicochemical properties. In: Fate of Chemicals in the Environment, American Chemical Society Symposium Series 225.
Publication date: Jan. 1, 1990
Revised: Sept. 28, 2023
AG-439-09
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.