The pH of the soil is a measurement of its acidic or alkaline status. Plants differ in their tolerance to soil pH as evidenced by the range of desired or target pH values of some crops grown in mineral soils of North Carolina (Table 1). Excessive soil acidity (pH < 5.2) in mineral soils is harmful to most crops, thus liming is a common practice to raise soil pH of agricultural fields. In some cases, however, it is necessary to lower soil pH. For example, blueberries grow best where pH is 5.0 or less, so lowering pH is necessary in many fields across North Carolina where pH has been managed for traditional agricultural row crop production. Excessively high pH is sometimes found where excessive rates of lime or other amendments, such as long-term poultry litter, have been applied. Naturally occurring shell fragments and marl in soils of some Coastal Plains counties can cause higher-than-desired pH for certain crops. In agricultural field crop production, the greatest concern with high pH is lowered micronutrient availability.
|
Crop |
Target pH |
|
Azalea |
5.0 |
|
Blueberry |
5.0 |
|
Centipede |
5.5 |
|
Fraser fir (established) |
5.5 |
|
Lawns (excluding centipede) |
6.0 |
|
Corn, soybean, small grain |
6.0 |
|
Cotton |
6.2 |
|
Vegetable garden |
6.5 |
|
Roses |
6.5 |
|
* Soil Testing Section, Agronomic Division—NC Dept. of Agriculture and Consumer Services (NCDA&CS). |
|
Understanding the Soil Cation Exchange Capacity (CEC), Base Saturation (BS), and Exchangeable Acidity (Ac)
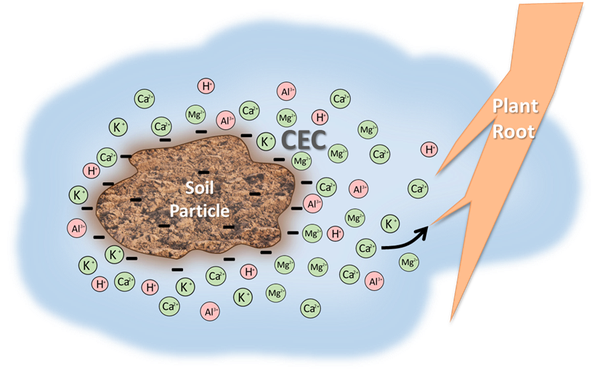
Clay particles and organic matter are negatively charged, thus they attract and hold positively charged elements called cations that are dissolved in soil water (Figure 1). Each soil type has a different storage capacity for cations, measured as the Cation Exchange Capacity (CEC) with units of milliequivalents per 100 cubic centimeters of soil (meq/100 cc). The CEC increases as amounts of organic matter and clay increase in the soil. Even though soils hold many different cations, generally more than 99 percent of the CEC is occupied by calcium (Ca), magnesium (Mg), potassium (K), aluminum (Al), and hydrogen (H).
The first three elements, Ca, Mg, and K, are nutrients required for plant growth. These are basic in nature, meaning no acidity is formed from their presence. The last two, H and Al, are toxic to plants in excessive amounts due to their acidic nature. Hence, the CEC is composed of both basic, plant-essential nutrients and acidic elements. The percentage of the CEC composed of Ca, Mg, and K is referred to as Base Saturation (BS). The remaining part of the CEC is occupied by H and Al. On a soil test report, the values of these acidic elements are reported as Exchangeable Acidity (Ac). A sample NCDA&CS soil analysis report can be found on page 3 of “Crop Fertilization Based on North Carolina Soil Tests”.
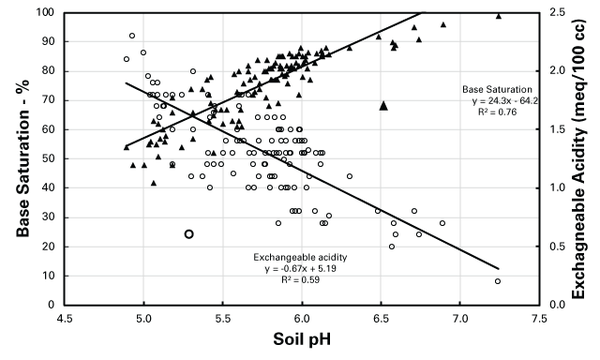
Soil pH values are positively correlated with BS and negatively correlated with Ac values (Figure 2). Generally, soil with lower pH has a higher Ac value and lower BS status as opposed to soil with a higher pH. When soil is limed, the alkaline reaction neutralizes acidity (H and Al), decreasing Ac and increasing the BS and soil pH. Conversely, to decrease the soil pH, it is necessary to apply an amendment to increase the acidity and thereby decrease the BS. At a similar soil pH, clayey soils have higher CECs, and thus their Ac values will be greater than sandy, low-CEC soils. Therefore, to change the soil pH in a clayey soil, more amendment material is needed than in sandy soil.
Materials Used to Decrease the Soil pH
Any material that produces hydrogen ions (H+) can be used to lower soil pH. The most effective materials are elemental sulfur (S0) and ammonium-based (NH4+) fertilizers. Table 2 shows the chemical reaction of these materials, generating H+ during their reaction in soil. The H+ replaces basic cations such as Ca, Mg, and K. Applying organic matter to soils can also lower the pH in some cases, but the reaction takes a long time to be effective. When slight modifications (0.5 pH units) are needed for individual plant installations, acidic material such as ground pine bark, using a volume of about one half of the total fill, is often successful.
Elemental sulfur is preferred to lower soil pH because it is inexpensive, is highest in acidification power, and produces sulfate (SO42-) which plants need. Sulfate may leach in sandy soils but can accumulate in significant quantities where clay increases with soil depth; subsoil reserves of sulfate often benefit deep-rooted plants. Losses through the soil system into groundwater and streams are usually harmless. Ammonium-based fertilizers can also be used to decrease pH. Unfortunately, the rate of ammonium required to lower pH is very high, often in excess of plant needs, and may result in nitrogen loss from the field and into surface and ground waters. Consequently, the use of ammonium fertilizers is recommended only when a slight pH reduction is needed (up to 0.3 pH units).
|
Acidifying Material |
Chemical formula |
Chemical Reaction (1) |
Acidification Value (lb/lb CaCO3)(2,3) |
|
Elemental Sulfur |
S0 |
2S0 + 2H2O + 3O2 → 2SO42-+ 4H+ |
0.32 |
|
Ammonium sulfate |
(NH4)2SO4 |
(NH4)2SO4 + 4O2 → SO42- + 2H2O + 2NO3- + 4H+ |
0.88 |
|
Monoammonium phosphate (MAP) |
(NH4)H2PO4 |
(NH4)H2PO4 + 2O2 → H2PO4- + H2O + NO3- + 2H+ |
1.67 |
|
Diammonium phosphate (DAP) |
(NH4)2HPO4 |
(NH4)2HPO4 + 4O2 → H2PO4- + 2H2O + 2NO3- + 3H+ |
1.54 |
|
Ammonium nitrate |
NH4NO3 |
NH4NO3 + 2O2 → 2NO3- + H2O + 2H+ |
1.61 |
|
(1) All chemical reactions presented need soil moisture and are mediated by soil microorganisms; |
|||
The soil acidification process depends on microorganisms’ activity and soil aeration, thus the reaction is slow in cold, wet soils. In normal conditions, it is expected that pH will decrease within three to six months after the application of elemental sulfur or ammonium-based fertilizers.
Calculating the Acidifier Requirement to Decrease the soil pH
To calculate the amount of Acidifier Requirement (AR) for a desired pH (Equation 1), the CEC and current BS percentages are needed from a current soil test report. To use Equation 1, obtain the value of the material’s Acidification Factor from Box 1 and obtain the value of the Target BS that corresponds to the desired soil pH from Box 2.
Equation 1:
AR (lb/acre) = CEC × ((Current BS - Target BS) ÷ 100) × AF
AR = Acidifier Requirement (lb/ac)
CEC = cation exchange capacity (meq/100 cm3), from the soil test report
Current BS = current base saturation percentage, from the soil test report
Target BS = target BS percentage based on desired soil pH (Box 1)
AF = Acidification Factor for the chosen material (Box 2)
|
Desired Soil pH |
Target BS Percentage |
||
|
Mineral |
Mineral-Organic |
Organic |
|
|
4.0 |
38 |
37 |
43 |
|
4.5 |
49 |
48 |
54 |
|
5.0 |
59 |
60 |
65 |
|
5.5 |
69 |
71 |
76 |
|
6.0 |
80 |
83 |
86 |
|
6.5 |
90 |
94 |
97 |
|
* Values of BS are in 702,764 soil samples from North Carolina |
|||
|
Acidifying Material |
AF* |
|
Elemental Sulfur |
320 |
|
Ammonium sulfate |
880 |
|
Monoammonium phosphate (MAP) |
1670 |
|
Diammonium phosphate (DAP) |
1540 |
|
Ammonium nitrate |
1610 |
|
* AF is based on Acidification Values plus conversion factors to obtain the result in lb/ac |
|
The output from Equation 1 is in pounds of acidifier material per acre. To calculate the rate required for small fields, convert the result from Equation 1 to lb/1000 sq ft by dividing the result by 43.6.
For example, a farmer has a field with a mineral-organic soil with the current soil test results: pH = 7.1, CEC = 5.7 meq/100cc, and BS = 97 percent. The farmer plans to grow blueberries in this field and desires to lower the soil pH to 5.0 using elemental sulfur. The calculation is: AR = 5.7 × ((97- 60) ÷ 100) × 320 = 675 lb/acre of elemental sulfur. The values “60” and “320” come from Box 1 and Box 2, respectively, because we chose to lower the pH to 5.0 (Box 1) using elemental sulfur (Box 2). Using the same example, but in units of lb/1000 sq ft for a lawn or garden, the calculation will be: AR = 5.7 × ((97- 60) ÷ 100) × 320 ÷ 43.6 = 15.5 lb/1000 sq ft.
Products and Safety Considerations
Elemental sulphur contains 90 percent sulphur and is available in powder and granular forms. For ease of handling, safety, and uniform application, the granular form is best. Skin and eye protection using personal protective equipment is warranted since the material is caustic. In addition, inhalation of dust is a hazard. Follow all user directions. Plant burn may occur. If so, wash off any foliage immediately. To avoid this issue, do not apply sulphur to wet plant foliage.
Ammonium products are granular. If applied by hand in a home situation, skin contact should be minimized, and plastic gloves are advised, as with use of any fertilizer.
Final Remarks
Ammonium-based fertilizer should never be applied at rates greater than crop nitrogen needs. When attempting to acidify the soil, it is imperative to incorporate the elemental sulfur or ammonium fertilizer in the top six inches of soil, otherwise excessive acidification will occur in the soil surface with limited effect to the actual root zone. The efficiency of these amendments is dependent on the soil characteristics and microbial activity. The decrease in pH will generally occur between three and six months after the application but may take longer. Soil sampling before planting is advised to determine the pH change required.
References
Pierre, W.H. 1928. “Nitrogenous Fertilizers and Soil Acidity: I. Effect of Various Nitrogenous Fertilizers on Soil Reaction.” Journal of the American SocIety of Agronomy 20, no. 3: 254-269.
Publication date: July 23, 2020
AG-439-88
Recommendations for the use of agricultural chemicals are included in this publication as a convenience to the reader. The use of brand names and any mention or listing of commercial products or services in this publication does not imply endorsement by NC State University or N.C. A&T State University nor discrimination against similar products or services not mentioned. Individuals who use agricultural chemicals are responsible for ensuring that the intended use complies with current regulations and conforms to the product label. Be sure to obtain current information about usage regulations and examine a current product label before applying any chemical. For assistance, contact your local N.C. Cooperative Extension county center.
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.