Why Care About Healthy Aquaculture?
- U.S. imported aquaculture was valued at over $18 billion in 2015, so enhanced domestic production impacts both federal and local economies1.
- Bivalve aquaculture is a sustainable source of food – production is projected to increase by 15.4% by 20302, decreasing the need for capture production.
- Living shorelines of shellfish are used as breakwaters to protect the coast3,4 and could potentially mitigate erosion and scour around bridges5,6,7 and other coastal infrastructure.
Demand for seafood has increased in tandem with global food insecurity, and aquaculture has gained popularity as a sustainable, cost-effective food source supplying over half of the world’s fish supply2,8. This supply continues to surpass wild-caught stock, due to environmental stressors and overconsumption of natural marine seafood8.
Bivalves (e.g., oysters and clams) are not only an important food commodity, but they are also resilient to environmental changes and contribute crucial ecosystem services to the coast. They serve as natural breakwaters, protecting their local habitat during increases in sea level, while also combating the effects of eutrophication and providing protected habitat for numerous other marine and terrestrial species.
Increased Sediment Deposits
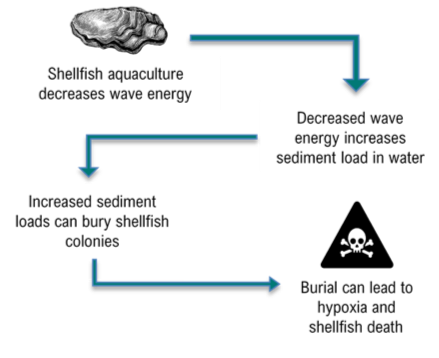
Sediment is resuspended at varying intensities near aquatic habitats by several events and human activities including: storms, freshets, and dredging9. Both engineered shellfish living shorelines and natural shellfish reefs protect the coast because of their ability to decrease wave energy impacts on land3,4. However, as this energy decreases, so does the capacity to transport sediment (Figure 1). This decreased transport capacity, in addition to resuspended sediment, means sediment is prone to settle and accumulates right where living shorelines are likely to exist.
In some cases, poor aquacultural management or harvesting practices can result in high amounts of sediment deposition and may contribute to reef failure or other ecological challenges10,11,12. For example, hydraulic dredging for clams (underwater clam harvesting) strips away the river bottom, which results in large sediment plumes. Increased sediment accumulation can partially or fully bury some shellfish colonies, resulting in deadly scenarios of hypoxia and mass shellfish mortality9. Even when the organisms are able to survive low oxygen conditions, burial disrupts their ability to feed and produce biodeposition (pseudofeces). Biodeposition is an important metabolic process that is key for sustaining reef habitat because it contributes to reef growth9. Therefore, a healthy synergy between sediment and aquaculture is needed to reduce the impacts of their relationship.
Bacterial Contamination
Currently the Food and Drug Administration (FDA) is mainly focused on limiting bacterial growth in seafood products for consumption. The implications of bacterial contamination can be serious, and they need to be further addressed with research and mitigation methods.
Sediment can contaminate bivalve aquaculture and compromise food safety through pollution and by carrying bacteria and pathogens. For example, the pathogen Vibrio vulnificus occurs naturally in water, but usually not at ambient temperatures. It can, however, become an issue at higher temperatures or in sediment around oyster habitats13. Vibrio vulnificus causes gastroenteritis, fatal septicemia, and other infections that could lead to limb amputation14,15 to humans that consume or are in close contact with affected oysters. These risks could potentially threaten beachgoers, shellfish consumers, and those who fish in affected areas.
Addressing the Risks
- Hydraulic dredging produces sediment plumes, agitating and releasing contaminants and pollutants. Clam harvesters should resist overharvesting according to their most recent local and NOAA regulations, and avoid dredging in submerged aquatic vegetation beds or near oyster sanctuaries.
- Oyster depuration (bacteria filtering process) has demonstrated the ability to decrease aquaculture bacterial contaminants,16,17,18 and should be used to purge oysters of any contaminants due to the presence of excess sediment.
- Coastal watershed planners should practice scour and erosion mitigation methods in streams and on the coast with practices such as living shorelines and riprap. Preventative planning will reduce the negative impacts of excess sediment on local bivalve aquaculture, and foster an environment for healthy, thriving colonies.
- Potential bivalve aquaculture farmers should use the North Carolina Shellfish Siting Tool, created and maintained by the University of North Carolina Wilmington. This visual tool informs potential shellfish farmers about potential excess sediment risks through data for particular areas in North Carolina: salinity, bottom type, depth soundings, shellfish growing area classifications, boat access areas, surrounding land cover and current shellfish growing operations.
References
1 National Oceanographic and Atmospheric Administration (NOAA). (2015). https://www.st.nmfs.noaa.gov/commercial-fisheries/fus/fus15/index, accessed last 21 February 2020.
2 FAO. (2018). The State of World Fisheries and Aquaculture 2018. Opportunities and challenges. Food and Agriculture Organization of the United Nations.
3 Hall, M. & S. (2018). Hydrodynamic effects on oyster aquaculture systems: A review. Reviews in Aquaculture. DOI: 10.1111/raq.12271
4 Hall, S., R. Beine, M. Campbell, T. Ortego, J.D. Risinger, 2017. Growing Living Shorelines and Ecological Services via Coastal Bioengineering in: Living Shorelines The Science and Management of Nature Based Coastal Protection (ed. Bilkovic et al.). ISBN 9781498740029
5 Hall, S., Beine, R., Campbell, M., Ortego, T., and Risinger, J.D. (2017). Growing Living Shorelines and Ecological Services via Coastal Bioengineering. In: Living Shorelines, The Science and Management of Nature Based Coastal Protection, Bilkovic, D., Mitchell, M., La Peyre, M., and Toft, J. (Eds), 249-270, CRC Press, ISBN 9781498740029.
6 Hughes, S.A. (2016). Scour and scour protection. http://www.oas.org/cdcm_train/courses/course4/chap_8.pdf; last accessed 6 June 2020.
7 García-March, J. R., García-Carrascosa, A. M., Cantero, A. P., & Wang, Y. G. (2007). Population structure, mortality and growth of Pinna nobilis Linnaeus, 1758 (Mollusca, Bivalvia) at different depths in Moraira bay (Alicante, Western Mediterranean). Marine Biology, 150(5), 861-871.
8 FAO. (2019). The State of World Fisheries and Aquaculture 2019. Meeting the Sustainable Development Goals. Food and Agriculture Organization of the United Nations.
9 Colden, A. M., & Lipcius, R. N. (2015). Lethal and sublethal effects of sediment burial on the eastern oyster Crassostrea virginica. Marine Ecology Progress Series, 527: 105-117.
10 Bahr L. M., & Lanier, W. P. (1981). The ecology of intertidal oyster reefs of the South Atlantic coast: a community profile. US Fish and Wildlife Service, Washington, DC.
11 Taylor, J., & Bushek, D. (2008). Intertidal oyster reefs can persist and function in a temperate North American Atlantic estuary. Mar Ecol. Prog. Ser. 361: 301−306.
12 Powers, S. P., Peterson, C.H., Grabowski, J.H., & Lenihan, H.S. (2009). Success of constructed oyster reefs in no-harvest sanctuaries: implications for restoration. Mar. Ecol. Prog. Ser. 389:159−170.
13 Chase, E., Young, S., & Harwood, V. J. (2015). Sediment and vegetation as reservoirs of Vibrio vulnificus in the Tampa Bay Estuary and Gulf of Mexico. Appl. Environ. Microbiol., 81(7), 2489-2494.
14 Muldrew, K.L., Miller, R.R., Kressin, M., Tang, & Y.W., Stratton, C. (2007). Necrotizing fasciitis from Vibrio vulnificus in a patient with undiagnosed hepatitis and cirrhosis. J. Clin. Microbiol. 45:1058-1062.
15 Gulig, P.A., Bourdage, K.L., & Starks, A.M., 2005. Molecular pathogenesis of Vibrio vulnificus. J. Microbiol. 43(Suppl):118 –131.
16 Phuvasate, S., Chen, M. H., & Su, Y. C. (2012). Reductions of Vibrio parahaemolyticus in Pacific Oysters (Crassostrea gigas) by depuration at various temperatures. Food microbiology, 31(1), 51-56.
17 Tokarskyy, O., Marshall, D. L., Dillon, J., & Andrews, L. S. (2019). Long-Term Depuration of Crassostrea virginica Oysters at Different Salinities and Temperatures Changes Vibrio vulnificus Counts and Microbiological Profile. Journal of food protection, 82(1), 22-29.
18. Ming, Z., Su, Y. C., DeWitt, C. M., & Waite‐Cusic, J. (2018). Flow rate of depuration system has minimal impact on Vibrio parahaemolyticus decontamination in Pacific oysters (Crassostrea gigas). Journal of food safety, 38(6), e12531.
Publication date: July 22, 2020
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.