Since HACCP was first introduced in the 1990s, designing and implementing a Hazard Analysis and Critical Control Point plan for shell eggs has become more complex than originally thought. New regulatory requirements have been added, including egg refrigeration and the Food and Drug Administration (FDA) Egg Safety Plan. Although these regulations address some food safety risks, it is important for egg operations to also conduct their own risk assessments due to continually emerging pathogens. Developing a HACCP plan is one way to evaluate risks and tie all the regulatory requirements and any additional risks into one food safety plan.
Production environment, egg temperature (initially and throughout processing and storage), and wash water pH and temperature play key roles in reducing microbial growth in shell eggs, and should be key in developing a HACCP plan for shell egg facilities. Board and Tranter (1995) noted that the surfaces of shell eggs can acquire bacteria from every surface they come in contact with. Shell eggs can also be cross contaminated by mechanical means from other materials or noncontact surfaces in the processing plant.
We have compiled data from published research related to shell egg facilities, which could be helpful when developing a HACCP plan. The goal of this publication is to provide a practical approach to developing and designing a HACCP plan. The step-by-step process will offer some tips on how to approach each step and provide resources that may be of assistance. One requirement for prerequisite programs not discussed in this publication is the expectation of a written sanitation standard operating procedure (SSOP) to be in place prior to the creation of a HACCP plan.
Steps to Create a HACCP Plan
The HACCP system is a recognized method to support production of a safe product. HACCP uses a preventive approach rather than a reactive approach. Each facility must develop an individualized plan because specific food safety hazards may vary among facilities depending on layout and design, equipment, sanitation procedures, personnel, environment, and product flow. We will discuss a step-by-step process for developing a model HACCP plan for individual facilities. The first step is to create a team of people who represent a cross section of diverse expertise and perspective from different areas in the operation.
The second step is to gather information about all current egg safety related programs currently in position. Key prerequisite programs should be in place to support a HACCP plan. Every operation should have a recall and traceability plan, SSOPs, preventive maintenance practices, employee training records, and a pest control plan. Any number of other prerequisite programs that build a food safety platform may also be created. Since HACCP cannot be an effective stand-alone food safety plan, these prerequisite programs are critical to ensuring a complete food safety process.
For the example plan presented, a receiving prerequisite program is needed. Noted in the hazard analysis of the sample plan is an example of a biological hazard at receiving off-line eggs. For a receiving prerequisite program to appropriately replace a critical control point (CCP), it must contain much of the same information that would be needed in a HACCP plan. In the example for receiving off-line egg biological hazards, the receiving prerequisite program must address how biological hazards are evaluated at the point of receiving. Because it takes too long to get a measurement for microbial analysis, an indirect measurement is necessary. In the case of the “Receiving Off-line Eggs” step, the biological hazards are Salmonella and Campylobacter. Temperature is one recognized way of controlling Salmonella growth; therefore, the temperature history and current temperature of incoming eggs should be considered when evaluating biological hazards for off-line eggs. Temperature of incoming eggs will vary from season to season and from operation to operation (Patterson et al. 2008). In off-line processing plants (where eggs come from off-premises), initial internal egg temperatures of 62°F to 68°F (16.7°C to 20°C) can occur after tempering. Although preprocessing coolers are generally kept between 40°F and 45°F, egg temperatures decline only slightly. The key task at receiving is to check the temperature history of the arrival eggs to ensure they have been held and transported at 45°F. Eggs must be refrigerated within 36 hours of lay. The length of time they are in the cooler and the temperature of the cooler are both important. In addition to requirements at receiving time, there may also be supplier specifications at other checkpoints.
As the HACCP committee for your facility begins its work, you will need to develop a flowchart of the product process. Create an outline of the entire process involved. Identify and list steps in the process where significant hazards (physical, chemical, and biological) can occur. Assess the hazard and describe the prevention measures you must implement to eliminate or reduce the hazard.
Develop a Flowchart
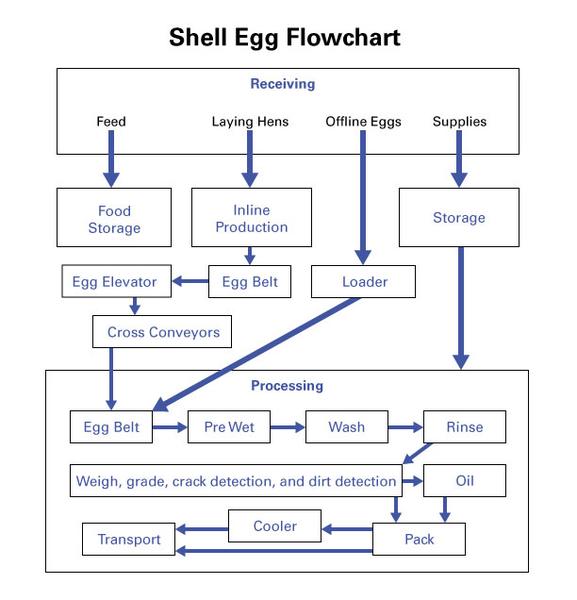
In the case of an egg facility, you must trace movement of eggs from the time they are received at your facility until they are loaded for distribution. The flowchart should include all areas of egg processing that you control. An effective strategy is to create a detailed flowchart similar to Figure 1. For in-line operations, the flowchart steps should begin in the production house and evaluate the movement of the eggs from the hens into the processing area. For off-line operations, the flowchart steps should begin whenever you acquire the eggs. Your hazard analysis should also consider off-line production hazards associated with the eggs you receive. Flowcharts should have individual steps: don’t group steps together.
HACCP Principle 1: Conduct a Hazard Analysis
Prior to starting your hazard analysis, acquire copies of any prerequisite plans you have in place. Include the specifications for receiving off-line eggs or your pre-harvest egg safety plan. You may also include other prerequisite programs such as sanitation programs, pest management programs, employee training programs, employee hygiene programs, specifications for purchasing materials (such as pallets, cartons, boxes, packing materials, cleaning supplies, and protective garments), laboratory tests conducted, and past food safety audits. All of these prerequisite programs can influence your determination of CCPs. Include only written prerequisite programs that contain monitoring results. You may need to reevaluate your prerequisite programs to ensure that they are being conducted appropriately and that you have documentation in place to show that they are being implemented as described. All hazards are reviewed in the hazard analysis. Any hazard that is not covered by a documented prerequisite program must be included in your HACCP plan.
For each step in the flowchart, you must evaluate biological, chemical, or physical hazards for that step. A biological hazard is the most commonly discussed type of hazard. A biological hazard includes organisms such as Salmonella and other pathogens. A chemical hazard could come from a cross contamination in the environment or a chemical passed to the egg from the feed, water, or sanitation chemical. A physical hazard in liquid eggs could include shell or metal shavings from process equipment.
The hazard evaluation determines if the hazard will cause potential harm to the person consuming the egg and is determined for each specific step in the process. You must determine if the hazard can be introduced, increased, or reduced to a nonhazardous level at that specific step and assume all other steps are being properly carried out. For each step in the flowchart, you must document biological, chemical, and physical hazards.
Start at the beginning of the flowchart. In the example provided, assume that the egg packing operations obtain eggs from both in-line and off-line production facilities. A sample hazard analysis has been provided.
Flowchart Steps
The following information provides some insight about the hazard analysis example. At receiving, you must evaluate all potential biological, chemical, or physical hazards coming into your operation. Here, your paperwork on specifications for incoming products plays an important role. Evaluate all potential hazards associated with anything coming into your operation. Some operations may have prerequisite programs that cover some hazards. For example, an operation might have a prerequisite receiving program that would address potential receiving hazards; if this is the case, the prerequisite program could offset the need for a CCP at receiving.
Incoming feed is an example of an incoming product to evaluate for hazard analysis. The specific hazards will depend on the feed ingredients and type of feed used. Consider what you know about your feed supplier’s food safety practices. What food safety hazards are associated with each of your feed ingredients? What food safety specifications do you have for your feed?
The primary food safety biological hazard for receiving feed is the presence of Salmonella. Salmonella in livestock feed is not considered to be high risk to the animals unless it contains a serotype that is pathogenic to that species. For poultry, S. pullorum, S. gallinarum, or S. enteritidis (SE) (CPG 690.800) are considered potentially pathogenic to layers. However, all Salmonella are pathogenic to humans, which is why a strong food safety program is critical to preventing outbreaks. Chemical hazards for receiving feed might include mycotoxin contamination and medication carryover. Mycotoxins that are considered harmful to animals or humans are subject to regulatory action based on action levels instituted by the FDA (CPG 683.100). Ingredients that are subject to action levels should be tested to ensure that the ingredient-receiving level at the feed mill meets FDA regulations. Though the ingredients are likely tested on a schedule and not in every load, there should be an approved supplier program in place at the feed mill, and weather patterns should be monitored for conditions favorable to mycotoxins. Another potential chemical hazard is medication carryover. Is the feed mill manufacturing medicated feeds? There should be written procedures and documentation in place (as in for sequencing and flushing) to minimize carryover into nonmedicated feeds.
The next flowchart step is receiving laying hens. The Egg Safety Rule covers this step and the remaining Salmonella prevention and control steps for production. The FDA enforces the Egg Safety Rule.
The next flowchart step is receiving off-line eggs. For an off-line operation, the flowchart steps will start with receiving the eggs. Receiving may begin at farm pick-up or receiving at the packing facility —wherever you first gain possession of the eggs. Regardless of where you receive your eggs — from a cage belt, nest box, or at the door of the packing facility — you must consider what hazards the eggs may have been already been exposed to. For off-line operations, you will need a set of written and documented specifications that you and your supplier have agreed upon to control hazards to the eggs prior to the receiving step. Eggs should come from farms with egg safety plans and that have been documented as environmentally negative, which is the acceptable results from their required testing.
The receiving supplies segment of the flowchart will vary greatly from operation to operation. The types of hazards will also vary based on the supplies you purchase. Typical types of supplies include sanitation products, packaging products, and maintenance and repair products. Each type of supply should receive its own hazard evaluation. Most supply purchases have specifications that address any potential hazards. Packaging materials should have specifications related to food grade products, letters of assurance, or Certificates of Analysis (COAs) from manufacturers. Sanitation products should be commercial grade products approved for use in shell egg operations. Lubricants and other maintenance products should be approved for use in shell egg facilities and documented.
Feed Storage
Feed is often stored either in bags or bulk. For larger operations, bulk feed is transferred into bins for storage and transported into the houses using augers. For smaller operations, feed may be delivered in bags and stored in a warehouse or other facility on the farm. Consider the type of storage when evaluating feed for safety. Feed that is stored in bins should be covered and not exposed to rain or humidity that could result in the growth of pathogens or mold. Feed that is stored in bags should be kept out of the elements and in a pest-free environment to prevent the growth of pathogens or mold. Feed should be completely emptied out of bins and properly labeled to ensure there is no co-mingling of diets that may contain medications. These precautions should be documented in writing.
Moving to the in-line production system hazard analysis, current thinking is that the production system — as well as the environment surrounding the facility — could potentially impact the safety of the eggs and your HACCP plan. As you evaluate potential hazards and develop control mechanisms, you must understand how they interact and how bacteria can move within the system. The solutions may be associated with regulations, the prerequisite programs, Good Manufacturing Practices (GMP), or SSOPs to mitigate the risks. Another concern, which has arisen with the in-line systems, is associated with the houses that are determined to be Salmonella-positive. The concern is isolating the house’s production but also preventing bacteria from moving through the complex via cross-collection belting. Is there a means of dry sanitation for these belts that would be beneficial throughout the complex regardless of the bacterial load in any house?
Egg production houses are located throughout the United States and in many climatic regions or zones. Migratory flyways could affect egg safety. At issue is how to keep the outdoor bacterial populations out of a poultry house. Changing the behavior of people is the most practical preventive step — assuming they change their mindset from disease prevention to a pathogen-control mentality. For example, they can change the clothes they wear prior to entry. Rodents, insects, and dust particles, however, come into a poultry house at will and to a greater or lesser extent depending on the season.
For in-line operations, housing systems will determine where you start your evaluation. Where is the egg laid? For example, is it a nest box or a floor area? For in-line operations, you should have a production related food safety plan in place. The production food safety plan could be a part of the overall HACCP plan, but it would probably be easier to manage a pre-harvest food safety plan and a post-harvest food safety plan separately.
As eggs enter the processing and packing portion of the operation, internal in-line egg temperatures can range from 88°F to 96°F (31.1°C to 35.6°C). In off-line processing plants, where eggs are brought in from off-premises, initial internal egg temperatures of 62°F to 68°F (16.7°C to 20°C) can occur after tempering. Egg temperatures at processing generally reflect initial internal temperatures because eggs are brought into the processing room and tempered until they are placed on the processing line. Egg temperature at processing is very important, as U.S. Department of Agriculture (USDA) regulations require that wash water temperature be 90°F or higher or at least 20°F warmer than the highest egg temperature, whichever is greater. These temperatures must be maintained throughout the cleaning cycle (Koelkebeck et al. 2008).
Regulations also require that wash water be changed at least every four hours or more often if needed to maintain sanitary conditions. When the difference between wash water temperature and egg temperature is ≥40°F, thermal checks and cracks increase, allowing surface microbes greater access to the interior of the egg.
Contact between wash water spray and eggs during processing causes internal egg temperature to increase. Although blow drying following washing causes a slight decrease in temperature, internal egg temperature generally rises throughout the process and can continue to rise for up to six hours after eggs are placed in a cooler.
According to USDA regulations, eggs cannot be immersed at any time. Eggs may be pre-wet prior to washing if sprayed with a continuous flow of water of similar temperature to that of the wash water, and that flow of water must drain away. Machines that recirculate wash water must have replacement water added continuously. Chlorine-sanitizing rinse water may be used as part of the replacement water. This includes quaternary compounds or sanitizing compounds recognized as equivalent to the concentration level of active chlorine. Chlorine or quaternary ammonium-sanitizing compounds may be used as part of replacement water provided they are compatible with the detergent. Only potable water may be used to wash eggs, and USDA requires a certificate to this effect (USDA 1991). Rate and extent of bacterial growth during storage is favored by washing eggs in water with less than two ppm iron, so it is important to monitor the iron content of the wash water. USDA suggests that water with iron content in excess of two ppm should not be used unless it is de-ironized (Baker and Bruce 1994). Iron contamination may influence microbial growth when iron penetrates shell membranes. As bacteria grow on membranes in an iron-rich environment, they can produce metabolic products that allow microorganisms to penetrate and diffuse into the albumen, providing a more favorable medium for microorganism growth.
The Canadian Food Inspection Agency (CFIA) provides operational guidelines for egg grading stations routinely in Canada to ensure that egg washing guides are followed. Guidelines include: maintenance of wash water at a temperature of 43°C to 46°C (110°F to 115°F); maintenance of wash water at pH 11.0 to 12.5; maintenance and routine cleaning of washers and their parts (for example, brushes and rollers); and complete change of wash water and cleaning of holding tanks every two to four hours (CFIA 2013). These guidelines were developed to eliminate pathogens that may be present in the wash water and to minimize microbial contamination of the washed eggs. At present, bacterial numbers in egg wash water are monitored to ensure that adequate sanitation is achieved. Total viable counts >1 to 5 cfu/ml are considered unacceptable (Bartlett et al. 1993). The United States currently has regulations governing wash water temperature and time between water changes in the tank.
Defoamers play an important role in egg washing. When defoamers are not dispensed properly, foam in the wash tanks builds up and eventually overflows. When foam spills from the tanks, it can interfere with water level detection and affect water temperature and pH.
After washing, the hot water rinse may contain chlorine or quaternary sanitizers that are compatible with the washing compound. Iodine sanitizing rinses may not be used as part of the replacement water (USDA 1991, 2012).
After the hot water rinse, filtered ambient air dries the eggs. At this point the surface temperature of the egg reaches approximately 95°F. Anderson et al. (1992, 2008) found that the internal temperature of eggs continues to rise due to high shell surface temperatures and candling lights. With new blood-detection devices, however, the heat from candling is eliminated. Five minutes after the eggs were processed, their temperature was 12°F to 14°F above their initial temperature.
Shell eggs may then be oiled to seal the pores, provided this operation avoids contamination of the product. Processing oil that has been previously used and which has become contaminated can be filtered and heat-treated at 180°F for three minutes prior to reuse.
At present, there is potential for contamination of Salmonella inside the egg if temperature is abused. Salmonella contamination could be attributed to trans-ovarian contact from a Salmonella-positive hen or cross contamination of the shell surface from collection or processing equipment. If equipment is not cleaned and maintained properly, the weigh, grade, crack, and dirt detection equipment — including the washing, drying, and candling unit operations that operate continuously each day — could contribute to surface contamination. Cleaning must include egg contact and noncontact surfaces and areas. Eggs detected as "dirties" at candling are typically routed through a rewash line located at the end of the packing head distribution, which takes the eggs to be rewashed through the entire packing head distribution system. Newer machines route eggs to the rewash line prior to the packing head distribution. Whereas older and some smaller operations at one time soaked eggs to soften dried fecal material, this practice is outdated and illegal: soaking eggs in water for as little as one minute can facilitate microbial penetration through the egg's shell.
Immediately after packing, the cartons or flats are placed in cases. The cases are palletized, then wrapped for stability. Though efficient, these packaging procedures cause high egg temperatures created during processing to be maintained for several days (Anderson et al. 2008). Industry surveys have shown that as much as a week is required for temperatures to dissipate due to processing under these conditions (Anderson et al. 1992), yet virtually everyone in the shell egg processing industry uses these or similar procedures. Such procedures create potential for growth of Salmonella, if present, due to the amount of time it takes for egg temperature to drop below 45°F. Refrigerated trailers should not be used as storage facilities for post-processing shell egg storage. These trailers were not designed to facilitate temperature reduction in a product but rather to maintain the internal temperature of a cooled product during delivery.
Federal law requires eggs to be stored at or below 45°F, however, state laws can and do supersede this requirement. Many states have laws requiring eggs to be stored at temperatures at or below 45°F, which would require a storage period prior to delivery.
Researchers have found that the growth rate of SE in eggs responds directly to the temperature at which the eggs were stored and that holding eggs at 40°F to 45°F reduced the heat resistance of SE. It has been suggested that refrigeration reduces the level of microbial multiplication in shell eggs and lowers the temperature at which the organism is killed during cooking (Denagamage et al. 2015). Thus there is adequate justification to store eggs at 40°F to 45°F.
Humidity in the storage environment is important in maintaining egg weight and preventing microbial growth. Storage relative humidity lower than 72% can cause weight loss and a corresponding increase in air cell size. The optimal humidity range during storage is 72–76%. Storage in relative humidity of 80% can promote microbial growth.
During transport, there is also a potential for growth of Salmonella inside the egg due to the egg being too warm if refrigeration trailers are not operating properly.
Before moving to the CCP step, you must highlight any hazards that were identified. You will analyze these identified hazards as a part of the CCP determination. You may control some of the identified hazards through prerequisite programs or as control points rather than CCPs.
Determining Critical Control Points
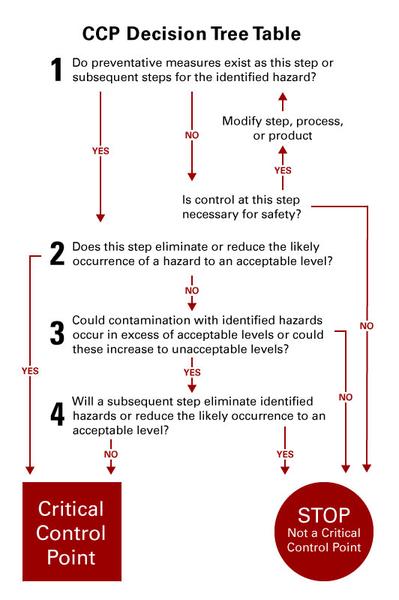
A CCP is a point, step, or procedure in the flowchart at which control can be applied and, as a result, a food safety hazard can be prevented, eliminated, or reduced to an acceptable level. Regulations have not prescribed a specific way in which CCPs must be identified. Decision trees (Figure 2) are sometimes useful, but if you participate in an auditing program such as Safe Quality Food (SQF) you could use their procedure for determining CCPs. Regardless of the procedure you use for determining the CCPs, remember that there is a difference in control points and CCPs. The CCPs are points in your operation where you must apply a control step or your product is potentially unsafe. A control point is where you take some action but if the action is missed, it does not impact product safety. A missed control point might impact quality, but it would not impact product safety.
It is important to differentiate between control points and CCPs. A good way to locate the CCPs is to begin with the first hazard identified at the first step of your process and then work your way through the process in the same path your product flows. In other words, go to the first step on your flowchart and determine which hazards are CCPs. Work with the hazards that you deemed significant during the hazard evaluation process. If an identified hazard is eliminated or reduced at a later process step or by normal consumer use, the raw material is not considered to have passed through a CCP.
Table 1 shows how the CCPs were determined both by using the risk/severity score and the decision tree. Only one of these approaches would be needed when you determine your CCPs. It is important to document why a hazard was not considered to be a CCP. For example, the hazard may be controlled through a prerequisite program or at a different step in the process.
Feed Receiving
The feed receiving step is part of the hazard analysis, but it may not become a CCP for the biological hazard because the presence of a Salmonella serotype that is pathogenic to poultry is likely very low. The feed receiving step is also not a CCP for the chemical hazard because testing for mycotoxins is generally the responsibility of the feed manufacturing facility and should be monitored based on FDA action (Compliance Policy Guide 683.100) or advisory levels (Guidance for Industry and FDA: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products used for Animal Feed, 2010). Additionally, the feed manufacturing facility should purchase ingredients from approved suppliers and monitor environmental conditions that are favorable for aflatoxin proliferation.
The feed receiving step is not a CCP for physical hazards because the likelihood of injury or illness to humans or animals is very low. The feed manufacturing facility should have steps in place (such as magnets or feed cleaners) to keep physical contaminants out of the final product.
Receiving off-line eggs is not a CCP for biological hazards because it is also covered by a regulatory requirement and a prerequisite receiving program. Receiving of off-line eggs could be handled as a CCP but it would probably be better handled under company prerequisite programs that include refrigeration regulation, receiving checkpoints, and supplier specifications, including egg age and temperature history.
When receiving supplies such as packing materials and cleaning supplies, in the past, biological and chemical hazards have been handled by supplier letters of guarantee (LOG). Now, however, COAs are increasingly accepted because they provide data and handling requirements that are covered in the receiving prerequisite plan.
Feed Storage
Feed storage does not become a CCP for a biological hazard because the likelihood there will be serotypes present in the feed that are pathogenic to poultry is very low. Part of an operation’s food safety system should include adequate cleanout of bins and feeders so the environment is not conducive to pathogen growth. Feed bins should remain covered to keep the bins and contents dry. Feed storage is not a CCP for chemical hazards, as mycotoxin levels should be monitored at the feed manufacturing facility. Feed storage is also not a CCP for the chemical hazard of drug carryover; such transmission could be mitigated by using a prerequisite program or standard operating procedure (SOP) to ensure that bins are cleaned out between different diets and that feed is stored in the correct bins. There is no CCP for feed stored in bags because these should be stored out of the elements and away from pests. A pest control program would be an adequate prerequisite program to keep rodents and birds from contaminating feed products.
Egg Processing and Receiving
A variety of potential hazards can be associated with egg processing facilities. The following are examples of what hazards may exist in your plant and why they may not become a CCP in your operation. All facilities are different and unidentified hazards may also exist. Use the decision tree (Figure 2) for your analysis.
- Storage of packing materials is not a CCP for biological or chemical hazards because storage practices are addressed by SSOPs and employee training programs.
- The in-line production egg belts are not a CCP for biological or chemical hazards because they are addressed by SSOPs.
- The in-line production egg elevators are not a CCP for biological and chemical hazards because egg elevators are addressed by SSOPs and employee training programs.
- The loader is not a CCP for a biological hazard because it is addressed by the SSOP program.
- The processing egg belt is not a CCP for a biological hazard because it is addressed by the SSOP program.
- The processing pre-wet is not a CCP for a biological hazard because it is addressed by the SSOP program.
- The processing wash is a CCP for a biological hazard because the wash water is recirculated with some amounts of replacement water added at four-hour intervals or as long as five hours if approved by the USDA. Due to the recirculation, there is potential for pathogen build-up in the water. Research shows that maintaining a pH above 10.0 will control microbial growth in the wash water (Holley, R. and M. Proulx 1986).
- The process rinse step is not a CCP for a biological hazard due to its low likelihood of occurrence.
- The processing oil step is not a CCP for a biological hazard due to regulatory requirements and the SSOP program.
- The processing weight, grade, crack and dirt detection step is not a CCP for a biological hazard due to the SSOP program.
- The processing pack step is not a CCP for a biological hazard because at this point in the process, steps would have been taken to control the hazard during the previous cooling programs and/or later transport prerequisite program.
- The egg rewash step could be a CCP for a biological hazard. Those eggs detected as "dirties" at candling are typically routed through a rewash line located at the end of the packing head distribution, which takes the eggs to be rewashed through the entire packing head distribution system. Increased temperatures for the egg due to going through the wash repeatedly will cause the internal egg temperature to rise, potentially causing growth of SE if present. This could be a CCP for a biological hazard unless an equipment modification pulls them out of the system prior to the packing head.
- The processing pack step is not a CCP for a biological hazard because there is nothing that can be done at this step that would not be controlled during the cooling and/or transport step.
- The processing cooler is a CCP for a biological hazard because if the cooler is not maintained at 45°F or below, pathogens may grow. Such maintenance is also a regulatory requirement, but due to the step’s role in controlling pathogens it should also be a CCP.
- The processing transport step is a CCP for a biological hazard because not all eggs go into the post-processing cooler but rather go from packing directly to transport. Although the eggs will most likely not cool to 45°F during transport, the refrigeration trailers must be maintained at 45°F or below ambient temperature to meet regulatory requirements and begin the egg cooling process. Wholesale buyers need to realize that the higher egg temperatures in these instances where the pack date is the previous day are due to the freshness of the egg and the fact that the eggs have not been in a cooler long enough to achieve an internal temperature of 45°F (Anderson et al. 2008). If the eggs are abused during transport (held at temperatures above 45°F as documented by the refrigerated truck temp log) this would indicate to the wholesale buyers that the load should be rejected.
Establish Critical Limits
A critical limit is the maximum or minimum value to which a biological, chemical, or physical hazard must be controlled at a CCP to prevent, eliminate, or reduce to an acceptable level the occurrence of the identified food safety hazard. For each CCP identified, a limit must be set to establish operational limits or boundaries. As long as the process remains within these limits, hazards are being controlled effectively. For example, a critical limit of pH 10.0 for the shell egg wash water may be used to control microbial cross contamination of shell eggs during the washing process. The pH of 10.0 suppresses bacteria growth and maintains safety of the water used to wash the eggs.
For operations subject to a HACCP plan, an identified CCP and critical limit set a regulation that the operation must meet to be in compliance. The operation identifies the CCP and limit, and the regulators ensure that the operations are following their HACCP plan.
The first CCP is egg washing or “processing wash.” This biological hazard will require an indirect measurement. Controlling water temperature and pH are two indirect methods that could be used to control Salmonella growth in the recirculating wash water. Most processors use wash water much hotter than the minimum 90°F. Koelkebeck et al. (2008) and a survey by Anderson et al. (1992) found that processors used wash water temperatures that averaged 115°F. In 1955, Hillerman reported that
wash water maintained at 115°F would increase internal egg temperature by 0.4°F/second. To use water temperature alone to control bacterial growth, the water temperature would have to be at least 122°F. Because this could cause unnecessary heating of the egg, manipulating pH is probably a better indirect method of reducing Salmonella growth. Alkaline cleaning formulations produce an initial pH in the wash water near 11.0, and pH during operation typically continues in the 10.0–11.0 range, unfavorable for growth of most bacteria (Moats 1978). However, Jones et al. (1995) isolated Salmonella Heidelberg from the shell of a commercial egg processed in water with a pH of 10.2. Two Canadian researchers, Holley and Proulx (1986), found Salmonella species were able to survive at 38°C and 42°C (100.4°F and 107.6°F) when wash water pH was less than or equal to 9.5. Alkaline pH has been reported to increase the sensitivity of Salmonella to heat (Anellis et al. 1954; Cotterill 1968). Kinner and Moats (1981) found that at pH 10.0 and 11.0, bacterial counts decreased regardless of water temperature. They reported that bacterial counts decreased at 50°C and 55°C (122°F and 131°F) regardless of pH. Laird et al. (1991) indicated, however, that current processing practices are not sufficient to prevent potential contamination of washed eggs with Listeria monocytogenes; their study showed that Listeria is isolated readily from egg processing environments, including wash water.
A number of research studies have shown that a pH of 10.0 to 11.0 or above is necessary to control bacteria. The pH is a relatively inexpensive variable to monitor and offers significant protection against such bacteria as SE.
Many shell egg processors have no idea what the pH of their wash water is. Often, those who do monitor pH measure it only at the start of a shift. The pH may be 10.0 or 11.0 at the beginning of the shift, but recycling wash water, overflow losses, and added replacement water all contribute to reduced pH levels. Detergents elevate the pH of egg washers and are dispensed, for the most part, in concentrations necessary to clean the eggshell. Processors cannot neglect the importance of maintaining a constant pH, which can be quickly lowered by the constant addition of rinse water. In dual tank systems, pH in each tank can be different depending on how the wash systems are connected. The detergents and detergent dispenser must be listed as approved for use on eggs in the current List of Proprietary Substances and Nonfood Compounds (USDA), FSIS, Miscellaneous Publication Number 1419. The best approach for reducing microbial populations in wash water tanks, and therefore on eggs, occurs when detergents are added in amounts sufficient to maintain a pH of 11.0.
The last two CCPs are to control Salmonella growth during storage and transport. It takes too long to measure Salmonella growth directly using microbial testing, so you have to measure it indirectly. An indirect method would be measuring how long and at what temperatures the eggs have been stored. The Egg Safety Rule states that shell eggs must be held or transported at 45°F ambient temperature beginning 36 hours after lay.
Monitoring
Monitoring is a planned sequence of observations or measurements to establish whether a CCP is under control and to produce an accurate record for future verification. All CCPs must be monitored to determine compliance with established critical limits. You will need to identify your lot size prior to developing your monitoring procedures. Lot size can be whatever the HACCP committee chooses. For example, one lot could equal one transport load of eggs, one hour’s production, or one day’s production. Keep in mind when choosing a lot size that it will affect the amount of “suspect product” you may have to deal with in case of corrective action. Suspect product is that which has been produced between the last monitoring action in which it can be documented that the critical limit was met and the monitoring action at which it is determined that the process has failed to meet your critical limit. You will need to dispose of suspect product or prove that the suspect product is safe.
Determining when, where, and how measurements will be taken and recorded is paramount, and thus data should be collected to answer a number of questions:
- How much does the factor vary over time?
- How variable are repeated measurements using a given method (precision)?
- How accurate is the measurement?
- At what point during processing do significant changes in the factor tend to occur?
Ideally, monitoring indicates when there is a trend toward a loss of control so that you can bring the process back into control before a deviation occurs. You must document monitoring procedures in the HACCP plan. The documentation must identify the best monitoring procedures, determine the frequency, determine test procedures for each monitoring activity, and create a plan for establishing records of the monitoring procedure(s). When writing monitoring procedures, identify the person responsible for monitoring and document training on monitoring procedures and recordkeeping. The HACCP team should also document how it determined the best procedure and the frequency of monitoring. You may need this documentation as a justification during an audit or inspection. Remember that a monitoring procedure must give immediate results in real time. You may use an indirect measurement procedure as long as you have verification tests to prove that the indirect measurement is an effective alternative.
For the “receiving off-line eggs” CCP, the ambient transport trailer temperature for each load of incoming eggs must be checked. There are a few possible ways to check. One would be to check the refrigerated truck temperature log, which would not only give you the arrival temperature but the transport history temperature as well. Another option would be to have the receiving personnel check the transport trailer ambient temperature upon arrival, prior to unloading the eggs. You could also check the reefer log for temperature history and the ambient trailer temperature for current temperature upon arrival.
For the “processing cooler” CCP, the ambient temperature can be monitored continuously or periodically. Our example in the appendix takes a periodic approach to monitoring. If a continuous approach is taken, this CCP could be replaced with a verification procedure.
The monitoring for the processing transport step would take place just prior to loading eggs into a transport trailer. The objective of the CCP is to make sure the transport trailer has been cooled down to 45°F prior to loading the eggs.
All of the monitors would need to go through documented training to ensure they know the procedures for each of the monitoring steps they will be conducting. At least one backup monitor for each CCP should also be trained.
Corrective Actions
A deviation occurs when there is a failure to meet a required critical limit for a CCP. When monitoring indicates a deviation from the CCP critical limit(s), you should take corrective action to bring the situation back into compliance and determine how to handle any product that may have been adulterated. Taking measurements and then not taking action as a result of these measurements is fruitless; it is also dangerous from a legal perspective because you have identified a problem and chosen to ignore it.
Deviations from critical limits indicate an out-of-control CCP. You should implement a corrective action immediately upon discovering the deviation.
In the case of the “receiving company owned off-line eggs” as a CCP, for a biological hazard, the operation will need to have written documentation of when a load should be rejected and any additional steps that would need to be taken if a shipment arrives where the ambient temperature exceeds 45°F. If a shipment arrives and the ambient temperature is above 45°F, a corrective action must be taken. The monitor will need to document the answers to key questions when making a corrective action.
- Determine the cause of the deviation. In this case, why did the ambient temperature exceed 45°F? Look at the temperature history and determine if the temperature rose, for example, because the door had been recently opened or if the amount of eggs and their temperature caused a rise in the ambient temperature.
- Determine how to bring the temperature back under control. One way would be to reduce the ambient temperature by unloading the eggs and re-cooling the transport trailer.
- Determine how the deviation can be prevented from happening again. Your solution will greatly depend on why the problem happened in the first place. The solution must be implemented and documented.
- Determine what should become of the eggs in the shipment in which the deviation occurred. The responsibility for making this decision should be predetermined so the monitor knows whom to ask. The person making decisions will need to consider the age of the egg, which could impact temperature, and the temperature history of the storage of the eggs from post-lay through transport. Documentation must show how much product is impacted, the date and time of the impact, and what was done with the eggs from the shipment.
For example, if you have a CCP requiring a minimum wash water pH of 11.0, a correction describes what action the employee should take if the pH is below 11.0. Add more detergent, for example. If so, what is the process for adding more detergent? How much more should be added? How does the employee know when the process is back under control? Also, remember that in a regulatory and legal sense, actions that are taken and not recorded do not exist! Furthermore, corrective actions must also specify the disposition of all product produced during the period the CCP was out of control.
Establish Recordkeeping Procedures
Records are written documentation of the facility’s compliance with its HACCP plan. They allow the facility to trace the history of its operations should a problem arise. If reviewed regularly, trends in monitoring results can provide early warnings to avoid deviations. In the event of a product recall, proper records could help identify and narrow the scope of the recall. If legal actions follow a recall, well maintained records can be good supportive evidence.
What type of records should be maintained? HACCP regulations require records be kept for HACCP plan development. These include hazard analysis, CCP determinations, setting of critical limits, handling of deviations, and results of verification and validation studies. HACCP implementation records must also be maintained. There is not a specific format for CCP records, but the following information must be included:
- Title and date of record
- Product identification
- Critical limit
- Monitor’s signature
- Reviewer’s signature
The record cannot be designed in a format in which the monitor just uses a checkmark to show that the critical limit was met. The monitor must enter a value. For example, if the critical limit is 45°F, the monitor must enter “45”.
You must also document that employees who will be completing or reviewing record forms have been appropriately trained. Equipment calibration records should also be a part of HACCP record files.
You must maintain a copy of any decision-making documents used in developing your HACCP plan. For example, if you used a scientific article as the basis for your critical limit, keep a copy of that article in your files.
The Food Safety and Inspection Service (FSIS) requires record review for each CCP prior to shipment of the product. Where practical, these CCP records should be reviewed by an individual who did not produce the original records. The purpose of the pre-shipment review is to ensure that all critical limits for the product were met prior to shipment.
How long do these records have to be maintained? For refrigerated products such as shell eggs, the product records must be maintained for one year. However, for frozen, preserved, or shelf stable products like frozen liquid whole egg, the records must be kept for two years. These records may be stored off-site if such records may be retrieved and provided on-site within 24 hours of request.
The responsible facility individual must sign and date the HACCP plan. This signature signifies that the facility accepts and will implement the HACCP plan. Each time the plan is reassessed or modified, a new signature and date must be provided. FSIS requires that reassessment occur at least annually.
Verification and Validation
Verification and validation activities are very different. It is possible to have a perfectly verified but invalid plan. Verification is a process to ensure the plan is being carried out as written. Validation is ensuring that the plan is effectively addressing food safety concerns.
Verification
Management personnel should be in charge of verifying that the system works. Verification might entail record reviews to ensure measurements are being taken and recorded at the appropriate times; it may also include an evaluation of the plan to ensure that the plant is operating in compliance with the HACCP plan. Your verification plan must also include a procedure for pre-shipment sign-off. Pre-shipment sign-off must include checking each lot of product to determine that all CCPs have been met before the packed eggs leave your operation.
You may also decide to set up a regular review audit for your HACCP plan. The person responsible for verifying that the HACCP plan is being carried out properly can conduct a verification procedure by reviewing records or watching a monitor conduct a monitoring procedure. This process may be part of your verification portion of the HACCP plan. You will just need to write up a schedule, process, and documentation procedure as a part of your verification plan.
An important component of verification is routine calibration of all equipment used in monitoring (for example, thermometers and pH meters). You must also include verification documents in your HACCP recordkeeping system.
A pH meter is used for the CCP at the processing wash step. Therefore, you will need to develop and write a verification step.
You will need a thermometer to monitor the critical limits for the processing cooler and processing transport biological hazard. Therefore, you will need to develop and write a calibration schedule as a verification step.
When you first set up your HACCP plan, as a part of verification, your operation will need to implement the HACCP plan collection data to verify that the plan can be performed effectively.
Validation
When validating a HACCP plan, consider the following questions:
- Have all the food safety hazards that are reasonably likely to occur been identified?
- Are the control measures in the plan capable of controlling the identified hazards?
- Are the critical limits scientifically sound?
- Are monitoring procedures and frequencies scientifically sound?
- Are deviations handled appropriately?
- How are decisions made on impacted product?
- Are root causes for deviations being identified?
- Are activities functioning as designed?
- Are records being generated and reviewed regularly?
- How are prerequisite programs integrated with the HACCP plan?
As a part of validation, you can conduct microbial testing. It is up to each operation to determine the best way to validate its plan to ensure it is effectively controlling identified hazards.
At the least, an operation should document scientific data used to set the critical limits. The operation should also be able to justify why the monitoring schedule and procedures are sufficient to find identified hazards.
Summary
Developing and designing a HACCP plan for shell eggs has become more complex than originally thought when the regulation was first introduced in the 1990s. Egg refrigeration and the Egg Safety Plan have been added as regulatory requirements. These regulations address some risks, but due to continually emerging pathogens, it is important for egg operations to conduct their own risk assessments besides ensuring compliance with these regulations. The egg operations that want to rigorously look for risks, however, are faced with the dilemma of reporting their findings if they discover something that has not yet caused a problem. The reporting requirement is a disincentive for operations to explore and test for nonregulated risks.
References
Anderson, K. E., F. T. Jones, and P. A. Curtis. 1992. Heat Loss from Commercially Packed Eggs in Post-Processing Coolers. Commercial Egg Special Report. Vol. 1, ER-1. Raleigh, NC: North Carolina Cooperative Extension Service.
Anderson, K. E., P. H. Patterson, K. W. Koelkebeck, M. J. Darre, J. B. Carey, D. U. Ahn, R. A. Ernst, D. R. Kuney, and D. R. Jones. 2008. “Temperature sequence of eggs from oviposition through distribution: Transportation Part 3.” Poultry Science 87, no. 6 (June): 1195-1201.
Anellis, A., J. Lubas, and M. M. Rayman. 1954. “Heat resistance in liquid eggs of some strains of the genus Salmonella.” Food Research 19 (January): 377-395.
Baker, R. C. and C. Bruce. 1994. “Effects of Processing on the Microbiology of Eggs.” In Microbiology of the Avian Egg, edited by R. G. Board and R. Fuller, 153-173. London: Chapman & Hall.
Bartlett, F. M., J. M. Laird, C. L. Addison and R. C. McKellar. 1993. “The analysis of egg wash water for the rapid assessment of microbiological quality.” Poultry Science 72, no. 8 (August): 1584–1591.
Board R. G. and H.S. Tranter. 1995. “The Microbiology of Eggs.” In Egg Science and Technology, edited by W. J. Stadelman and O. J. Cotterill, 81-103. New York: Food Products Press.
Canadian Food Inspection Agency. 2013. Shell Egg Manual. Chapter 18 – Technical Information.
Cotterill, O. J. 1968. “Equivalent pasteurization temperatures to kill Salmonellae in liquid egg white at various pH levels.” Poultry Science 47, no. 2 (March): 354–365.
Denagamage, T., B. Jayarao, P. Patterson, E. Wallner-Pendleton, and S. Kariyawasam. 2015. "Risk factors associated with Salmonella in laying hen farms: Systematic review of observational studies." Avian Diseases 59, no. 2 (June): 91-302.
Hillerman, J. P. 1955. “Quick cooling for better eggs.” Pacific Poultryman: 18-20.
Holley, R. A. and M. Proulx. 1986. “Use of egg washwater pH to prevent survival of Salmonella at moderate temperatures.” Poultry Science 65, no. 5 (May): 922–928.
Jones, F. T., D. V. Rives, and J. B. Carey. 1995. “Salmonella contamination in commercial eggs and an egg production facility.” Poultry Science 74, no. 4 (April): 753–757.
Kinner. J. A. and W. A. Moats. 1981. “Effect of temperature, pH and detergent on survival of bacteria associated with shell eggs.” Poultry Science 60, no. 4 (April): 761-767.
Koelkebeck, K. W., P. H. Patterson, K. E. Anderson, M. J. Darre, J. B. Carey, D. U. Ahn, R. A. Ernst, D. R. Kuney, and D. Jones. 2008. “Temperature sequence of eggs from oviposition through distribution: Processing Part 2.” Poultry Science 87, no. 6 (June): 1187-1194.
Laird, J. M., F. M. Bartlett, and R. C. McKellar. 1991. “Survival of Listeria monocytogenes in egg washwater.” International Journal of Food Microbiology 12, nos. 2-3 (February): 115-122. https://doi.org/10.1016/0168-1605(91)90060-3.
Moats: W. A. 1978. “Egg washing—a review.” Journal of Food Protection 41, no. 11 (November): 919-925.
Patterson, P. H., K. W. Koelkebeck , K. E. Anderson, M. J. Darre, J. B. Carey, D. U. Ahn, R. A. Ernst, D. R. Kuney, and D. R. Jones. 2008. “Temperature sequence of eggs from oviposition through sequence of eggs from oviposition through distribution: Production—Part 1.” Poultry Science 87, no. 6 (June): 1182-1186.
U.S. Department of Agriculture—Agricultural Marketing Service, Livestock, Poultry and Seed Program. 2012. Shell Egg Graders Handbook: AMS-PY Instruction No. 910 (Shell Eggs) – 1. Washington, DC: Poultry Grading Division.
U.S. Department of Agriculture—Agricultural Research Service, Poultry Division. 1991. Regulations Governing the Grading of Shell Eggs and United States Standards, Grades, and Weight Classes for Shell Eggs. 7 Code of Federal Regulations Part 56. Office of the Federal Register, National Archives and Records Administration. Washington, DC: U.S. Government Printing Office.
U.S. Department of Agriculture—Food Safety and Inspection Service. 1997. HACCP Principles & Application Guidelines, adopted August 14, 1997; Content current as of 12/19/2017, National Advisory Committee on Microbiological Criteria for Foods.
U.S. Department of Agriculture—Food Safety and Inspection Service. 2019. List of Proprietary Substances and Nonfood Compounds. Miscellaneous Publication Number 1419.
U.S. Food and Drug Administration. 2010. Guidance Document. Guidance for Industry and FDA: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products used for Animal Feed.
Publication date: Oct. 21, 2019
Reviewed/Revised: Sept. 11, 2024
AG-862
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.