Inside
Introduction
Ethanol is becoming a popular alternative fuel source because it can be domestically produced from renewable materials. Its rise in popularity coincides with growing concerns surrounding petroleum supplies. Sharp increases in oil and gasoline prices and the problems associated with importing petroleum from unstable regions has led to a rapid increase in biofuel production in the United States. Ethanol is a biofuel that functions as a substitute for gasoline. As ethanol becomes increasingly available, consumers need a general understanding of how this fuel may impact their daily lives and relieve high energy prices.
What is Ethanol?
Ethanol is an alcohol-based organic compound produced chemically by ethylene conversion (a patented process) or through fermentation of sugars using yeasts. Ethanol (C2H5OH) is flammable, colorless, and odorless. It is a renewable alternative fuel for spark-ignition engines that typically use gasoline. Ethanol can be produced domestically. The majority of ethanol in the United States is produced from corn. Sugarcane, sugar beets, sweetpotatoes, and wheat are examples of other crops that can be liquified, fermented, and distilled to produce ethanol.
Besides its use as motor fuel, ethanol is used to produce vinegar and a variety of high-value industrial compounds. It is a popular solvent, extractant, and antifreeze. Ethanol can be found in perfumes, paint, pharmaceuticals, and anti-bacterial hand sanitizers.
Does Ethanol Use Benefit the Environment?
Yes. Pure ethanol is nontoxic, water-soluble, and biodegradable. It poses less of a threat to the environment than petroleum-based products because it will biodegrade if spilled in surface water, groundwater, or soil. When oxygen is present, spilled ethanol will simply convert to water and carbon dioxide.
Ethanol is also being used to replace methyl t-butyl ether (MTBE) as an oxygenate in reformulated gasoline blends. MTBE is very difficult to remove from groundwater and harmful to human health. Ethanol also has the potential to reduce greenhouse gas emissions from automobiles by as much as 30 percent when compared to gasoline. Carbon monoxide (CO) is the primary engine emission affected. Studies also indicate that a gallon of ethanol requires 40 percent less fossil fuel to produce and distribute than a gallon of gasoline.
What Is E85?
As gas stations across America begin offering alternative fuels, consumers need to understand what these fuels contain so they can choose wisely. Ethanol is produced at a processing plant called a biorefinery, where the feedstocks are liquified, fermented, and distilled into ethanol. At the end of the process, ethanol is similar to drinking alcohol. It is then blended with a denaturant—a substance used to make it unfit for drinking. For ethanol used as automobile fuel, the denaturant is usually a small amount of unleaded fuel: 4.7 percent of the total mix. The biorefinery then sends the denatured ethanol to a gasoline distributor, where it is blended with gasoline. Depending on the percentage of ethanol added to the unleaded gasoline, a new fuel product is created. For example, E85 is a mixture of 85 percent denatured ethanol and 15 percent unleaded gasoline. E85 is an alternative motor fuel for spark-ignition gasoline engines.
Likewise, E10 is a blend of 10 percent ethanol and 90 percent unleaded gasoline. E10 is not considered to be an alternative fuel because the ethanol is used to increase the octane level of the fuel and thereby reduce greenhouse gas emissions from automotive engines. E10 and E85 are the two most common types of ethanol blends on the market today.
Did You Know?
Adding ethanol increases the octane rating of regular unleaded gasoline.
Higher octane fuels will combust more efficiently, help reduce harmful emissions, and diminish engine knocking. Burning ethanol blends in older engines will loosen harmful deposits in the engine and fuel system. This may require replacement of the vehicle’s fuel filters if they become clogged with these deposits.
Can I Burn Ethanol in My Car?
Yes, but not all blends. Ethanol can be burned in any regular spark-ignition gasoline engine, but modifications to the engine may be necessary. Typically, E10 ethanol-gasoline blends will not harm an engine. E10 is a very popular oxygenated fuel blend. Because E10 helps reduce greenhouse gas emissions, the Environmental Protection Agency (EPA) mandates its sale in many urban areas.
As the amount of ethanol increases within the ethanol-gasoline blend, precautions must be taken. An automobile engine must be modified to use E85 blends for two primary reasons: First, ethanol is corrosive and will destroy the rubber hoses and gaskets found in a vehicle’s engine. Second, ethanol increases the octane rating of fuel. This causes a more intense explosion to occur in each cylinder. To prevent damage to the engine, the fuel-to-air ratio must be modified for engines that burn E85 fuel.
Drivers who want to burn higher blends of ethanol like E85 have two options: a flex-fuel vehicle or a conversion kit for an existing vehicle.
Many automotive manufacturers are currently offering flex-fuel or dual-fuel vehicles. These vehicles can burn either unleaded gasoline or E85 because their fuel lines have sensors that determine how much ethanol is present in the fuel and adjust engine parameters accordingly. Consumers usually do not pay extra for the flex-fuel option because automobile companies receive large tax credits for manufacturing these vehicles.
For older vehicles, “third-party” conversion kits are available that allow conventional engines to burn higher ethanol blends. However, the EPA must certify these kits to ensure the modification will not increase the vehicle’s emissions. Installing an unapproved conversion kit violates federal law and is subject to penalty.
Will Ethanol Change My Gas Mileage?
Yes. A gallon of ethanol does not contain as much energy as a gallon of gasoline. In other words, ethanol is less energy-dense than regular unleaded gasoline.
The British Thermal Unit (BTU) is used to measure how much energy is contained within a substance. A BTU is the amount of energy required to raise the temperature of 1 pound of water 1ºF at sea level.
Ethanol contains 76,000 BTUs per gallon, which is less than the 114,000 BTUs per gallon found in gasoline. With an E10 fuel, the drop in gas mileage will probably not be noticeable. With reformulation of the gas blend, generally a 1 to 2 percent drop in gas mileage is experienced when E10 is used to fuel a car versus unleaded gasoline.
E85 on the other hand will cause a noticeable drop in fuel economy. When E85 is used in flex-fuel vehicles, the fuel economy drops 20 to 30 percent depending on the vehicle make and model. This is because a gallon of E85 contains approximately 28 percent less energy than a gallon of regular gasoline. The unleaded gasoline and E85 fuel economy values for all flex-fuel vehicles can be found online.
Is Ethanol Cheaper Than Gasoline?
Yes and no. Typically, a gallon of E85 is noticeably cheaper than a gallon of unleaded gasoline. While initial savings may occur at the pump, the lower energy value for the E85 will result in more fill-ups, ultimately costing the consumer more money.
For instance, consider a pick-up truck with a fuel economy rating of 16 miles per gallon (mpg) in the city when operating on regular unleaded fuel. The fuel mileage on this truck will drop to around 12 mpg when E85 is burned. To drive this truck 1,000 miles on E85 instead of gas, an additional 21 gallons of fuel will be purchased. Because E85 has 72 percent of the energy value of gasoline, the price of ethanol should be 72 percent of the price of gasoline for the consumer to save money. So if gasoline is $3 per gallon, the E85 should be $2.16 per gallon or less to save the consumer money.
Also, the price of ethanol is strongly influenced by gasoline prices. When gasoline prices increase, so do ethanol prices.
How Much Corn Does It Take to Fuel a Car?
The best way to answer this question is to work through a little math. First, 1 bushel of corn produces around 2.7 gallons of ethanol. The average North Carolina corn yield is approximately 137 bushels per acre, so 370 gallons of ethanol can be produced from an acre of corn.
Now consider a midsized flex-fuel sedan that will be driven 12,000 miles per year. The E85 overall fuel economy value for this vehicle is 18 mpg. To drive the 12,000 miles, 667 gallons of motor fuel will need to be purchased. Remember E85 is only 85 percent ethanol, so 100 gallons of the fuel purchased will be unleaded gasoline. The remaining 567 gallons of ethanol will require the production of 210 bushels of corn. Assuming an average yield, 210 bushels can be produced on 1.55 acres.
Did You Know?
A bushel of corn weighs 56 pounds.
A single bushel of corn produces 2.7 gallons of ethanol and 18 pounds of distiller’s grain that can be used as livestock and poultry feed.
Where Is Ethanol Produced?
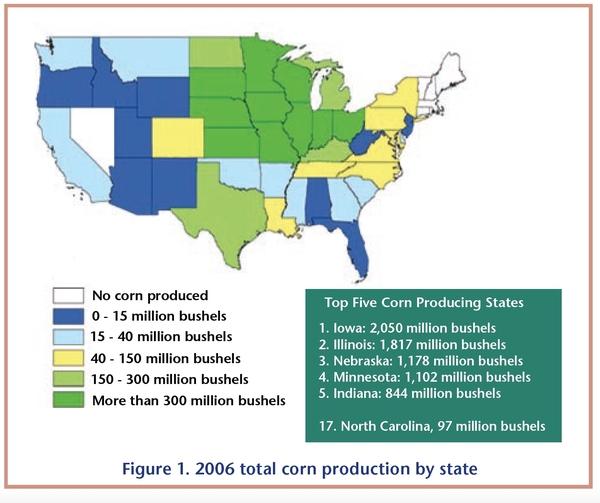
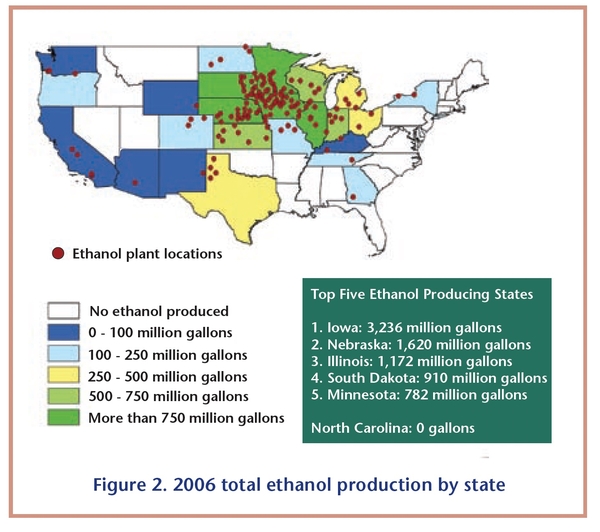
Because corn is the primary raw material used to make ethanol, the majority of ethanol production facilities are located in the U.S. cornbelt. Figure 1 and Figure 2 illustrate how closely the ethanol industry is associated with states that produce large amounts of corn. The top five corn-producing states account for nearly 67 percent of total U.S. corn production and nearly 64 percent of total U.S. ethanol production capacity. Production capacity includes all plants in operation, under construction, or expanding as of January 2007. Some states outside the cornbelt, such as California and Colorado, use locally available feedstocks, such as cheese whey and brewerywaste, to produce ethanol.
How Much Ethanol Can the U.S. Corn Crop Make?
The short answer is no one really knows.
Right now, there are only forecasts and speculation about how much ethanol will be produced as new technologies come on line.
Corn has limited capacity when it comes to ethanol production because it is an important human and animal feed. The U.S. Department of Transportation estimates annual U.S. gasoline consumption has surpassed 140 billion gallons. If the entire 2006 U.S. corn crop (approximately 10.5 billion bushels of corn) were converted to ethanol, the result would be 28 billion gallons of ethanol. Because ethanol has 67 percent of the energy content of gasoline, ethanol production would displace 19 billion gallons of gasoline or 13.5 percent of total U.S. gasoline consumption.
The National Corn Growers Association projects 15 billion bushels of corn and 15 billion gallons of ethanol will be produced by 2015. The projection considers stable grain markets, enhanced conversion technology, and improved corn yields. In 2006, the 106 biorefineries operating in the U.S. surpassed 5 billion gallons in annual ethanol production. Once all of the plants that are expanding or under construction in 2006 are completed, the U.S. will have the capacity to produce more than 8.5 billion gallons of ethanol a year.
| Feedstock | Ethanol Yield in Gal/Acre |
|---|---|
| Wheat | 340 |
| Corn | 400 |
| Sweet Sorghum | 600 |
| Sweetpotato | 640 |
| Sugarcane | 650 |
| Sugar beets | 700 |
| Switchgrass | 1000 |
| Miscanthus | 1250 |
Trends in U.S. Ethanol Production
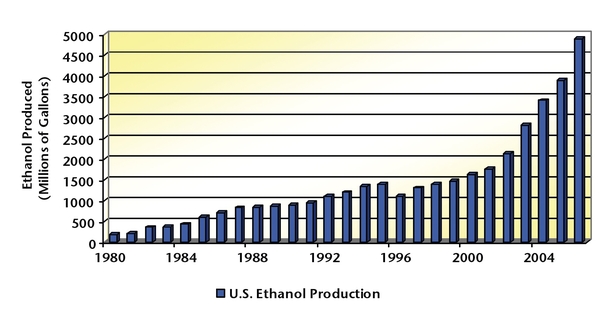
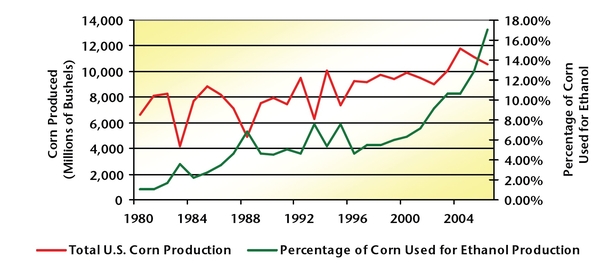
In the last 10 years, U.S. corn production has remained relatively stable, with an average annual production of slightly less than 10 billion bushels. The demand for corn has also remained fairly constant over that period, as the animal feed, human food, and international export markets have been flat. Over the last 5 years, ethanol production has witnessed a dramatic increase because of soaring energy prices, particularly with energy sources associated with petroleum (Figure 3). In 2006, ethanol production consumed nearly 18 percent of the total U.S. corn crop (more than 10.5 billion bushels) (Figure 4).The demands that ethanol has placed on the corn market is beginning to affect grain prices.
Some people worry that we will need to choose between using corn for motor fuel or food. Fortunately, the science of processing corn into ethanol is constantly evolving as improvements are made in conversion efficiency and corn production. For instance, U.S. corn yields have historically increased by 2 bushels per acre per year due to improved breeding and genetics. Also, seed companies are beginning to introduce corn varieties with high starch content for producing ethanol. With these special corn varieties and better conversion processes, more than 3 gallons of ethanol could be produced from a single bushel of corn. The National Corn Growers Association projects that the average ethanol yield will increase from 436 gallons per acre in 2004 to 587 gallons per acre in 2014.
Are There Alternatives to Corn?
Yes. While over 97 percent of the ethanol commercially produced in the United States is made from corn, other raw materials are easily converted to ethanol. Brazil, one of the world’s largest ethanol producers, uses sugarcane for most of its ethanol production. Sugarcane is easier to convert to ethanol than corn or other starch crops like potatoes. Wheat, barley, grain sorghum, and milo are other grains used in the U.S. for commercial ethanol production.
It is important for North Carolina to identify noncorn feedstocks for ethanol production. North Carolina is a grain-deficit state because the state does not produce enough grain to meet its in-state demands. Large amounts of grain are imported into the state to feed the extensive livestock and poultry operations. Potential noncorn ethanol feedstocks include trees, sweetpotatoes, and cotton stalks. Researchers are currently working to find methods to efficiently convert these plant materials into ethanol.
A new type of agricultural sector is developing with a focus on energy crop production. Energy crops would be produced by a farmer for the sole purpose of feeding a biorefinery. Potential energy crops include sweet sorghum, switchgrass, miscanthus, and algae.
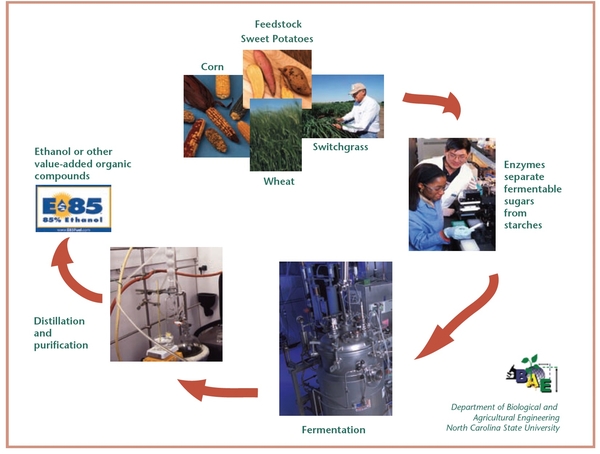
The Ethanol Production Process
Ethanol is produced from biological materials by converting sugars to alcohol through the process of fermentation and then distilling the fermented solution to separate the ethanol. These two basic processes remain the same regardless of the type of raw material (feedstock) used as the source of ethanol conversion. Additional steps may be added before fermentation, depending on the type of feedstock, to free the fermentable sugars from other organic compounds. Figure 5 illustrates how starches are converted to ethanol. This is the most common ethanol production process in the United States.
Fermentation
Fermentation is a process that occurs in an environment without oxygen present (an anaerobic environment) where yeasts break down sugars into simpler compounds. Yeast is a microscopic fungus that metabolizes carbohydrates (sugars) to produce other compounds. In the case of ethanol production, yeasts convert sugar into ethanol and carbon dioxide (CO2).
Distillation
Distillation is a process of separating various compounds based on differences in their boiling points. To use ethanol for fuel, water and ethanol must be separated after fermentation. At sea level, ethanol boils at 173°F and water at 212°F. If the water-ethanol mixture is heated to a temperature between 173°F and 212°F, the ethanol will turn into a gas and the water will remain a liquid. The ethanol gas will evaporate, thereby separating itself from the water. The ethanol vapors are then captured, cooled, and condensed back into a liquid. This process removes approximately 90 percent of the water in the ethanol-water mixture. Additional filtration is required to remove even more water if the ethanol will be used as a motor fuel.
Enzymatic Activity
Enzymes are proteins that trigger chemical reactions in biological cells. Enzymes react with the original, unprocessed material, known as a substrate, and convert it into multiple molecules, known as the products. Enzymes are needed in ethanol production to break down starches into sugar (glucose). Specific enzymes are developed to perform specific functions. Amylase is the primary enzyme used to break starches down into sugars. One of the factors preventing the introduction of additional starch feedstocks (such as wheat, barley, and sweetpotatoes) into the ethanol production process is identifying enzymes that will efficiently break down each plant’s starch compounds into sugars.
Cellulosic Conversion
Cellulosic ethanol is produced by converting the cellulose found in plant cell walls into ethanol. This conversion process is very complicated as additional steps are required. A pretreatment step is needed to separate the cellulose from other plant cell compounds, such as lignin. Also, a hydrolysis operation is needed to separate fermentable sugars from the cellulose. Currently, research is being conducted to find an economic and efficient way to perform pretreatment and hydrolysis on a variety of cellulosic feedstocks, which include switchgrass, cotton stalks, trees, and corn stover (stalks and leaves). Cellulosic-based ethanol holds the greatest hope for providing sustainable energy for the United States as well as having the greatest potential impact on North Carolina’s agricultural economy. The general opinion of most researchers is that cellulosic conversion is still 5 to 10 years from becoming a reality.
Ethanol is hydrophilic,
which means it is strongly attracted to water, making it difficult to purify for use as a motor fuel.
Is Ethanol the Only Product of Fermentation?
No. The fermentation and distillation of biological materials can create a wide variety of value-added chemicals. Many of these chemicals have greater monetary value than the ethanol used for motor fuel. Lactic acid is an example of a value-added product that is derived from fermentation. Lactic acid plays a critical role in food preservatives. Acetone, commonly used in nail polish remover and paint thinners, can also be derived by fermenting corn with special bacteria.
One of the co-products of fermentation is the large amount of carbon dioxide (CO2) that is released by the yeast as it breaks down sugar. By capturing, purifying, and condensing the CO2, many ethanol biorefineries improve their prifitability by selling this co-product to food and beverage companies.
Is Ethanol Production Energy Sustainable?
Yes. Analysts continue to discuss the energy balance associated with ethanol production. The critical question is, will more energy be consumed in producing ethanol than that contained in the final product?
When ethanol production was first developing, it took more energy to grow, harvest, process, and distill corn than that contained in the final product. As ethanol conversion technology has progressed, this balance has steadily improved. A conservative estimate shows ethanol production produces a net 25 percent increase in energy. For every 100 BTUs put into growing and converting corn to ethanol, 125 BTUs of energy are generated from the ethanol. As the conversion efficiency, farming practices, and transportation technology continue to improve, many analysts predict a 65 percent net increase in energy is possible.
Did You Know?
Brazil has the largest, most established commercial ethanol production industry in the world.
Nearly, 80 percent of all vehicles sold in 2005 in Brazil are flex-fuel. Brazil produces enough ethanol to offset 40 percent of its oil consumption, and many Brazilian cars burn a fuel known as gasohol—a blend of 75 percent gasoline and 25 percent ethanol.
Can I Make My Own Ethanol?
Probably not. Ethanol production is a difficult process that poses two major drawbacks for small-scale production. First, ethanol is a hydrophilic compound. This means ethanol is strongly attracted to water, making it very difficult to remove water during the distillation process. Most ethanol burning engines in the U.S require anhydrous ethanol, meaning there is very little water mixed with the ethanol. The required distillation process is currently expensive and difficult to implement on a small-scale.
Second, federal and state agencies do not distinguish between the manufacture of alcohol for human consumption and ethanol for fuel. This means ethanol production requires permitting from various agencies that govern the production of alcoholic beverages. Also, pure ethanol, like drinking alcohol, is subject to heavy excise taxes.
The consumer should be wary of buying homemade ethanol unless it has been tested and meets the American Society of Testing and Materials (ASTM) specifications for fuel grade ethanol.
Is Ethanol the Same as Drinking Alcohol?
Basically, yes. Ethanol, also known as ethyl alcohol, is the same chemical compound found in many alcoholic beverages. The processing required to make both of theses liquids is very similar.
The basic fermentation and distillation steps are needed to create the alcohol, but the production process varies depending on the final intended use.
The difference in processing occurs in the distillation and filtering operations near the end of the production cycle.Because alcoholic beverages will be consumed by people, the overall processing is more sterile and additional filtering is carried out to purify the ethanol. This additional purification can add up to $0.50 per gallon to the cost of producing drinking alcohol versus fuel alcohol.Ethanol is processed just to point that it can be used as a fuel; therefore, ethanol is cheaper to produce than drinking alcohol.
Fuel ethanol is also denatured with a substance that will make it either toxic or taste bad to prevent people from drinking it. Denaturing the ethanol relieves ethanol producers from heavy excise taxes that most states or localities place on alcoholic beverages, including pure ethanol.
What Are the By-products of Production?
When ethanol is produced, two important by-products are created: distiller’s grain and carbon dioxide.
Distiller’s grain is the fraction of the corn kernel that remains after the starch is converted to ethanol. Distiller’s grain is made up of proteins, fat, vitamins, and minerals found naturally in the corn kernel. About 18 pounds of distiller’s grain is produced for each bushel of corn converted to ethanol.
Distiller’s grain is a valuable livestock and poultry feed. Carbon dioxide is a by-product of the fermentation process. The CO2 released by the yeasts can be captured and sold for a variety of purposes. It is filtered to remove residual alcohols and compressed. It can then be sold to carbonate beverages, create dry ice, flash-freeze meats, and serve critical needs in the food processing and paper industries.
Are There Ethanol-Related Tax Incentives?
Yes. Ethanol affects taxes on a variety of levels. For the consumer, however, the tax impact is minor. The majority of the tax incentives focus on the development of a stable, domestic ethanol industry. The following three tax strategies support the growth of ethanol in the United States:
- Ethanol is subsidized by the federal government. The fuel blender receives a tax credit of $0.51 for every gallon of ethanol blended into gasoline. This credit is only available to the company responsible for mixing the ethanol and gasoline.
- The U.S. sets high tariffs on ethanol produced overseas to discourage ethanol imports.
- Automobile manufacturers receive tax credits for building automobiles that will run on alternative fuels. This incentive helps to keep the cost of alternative vehicles comparable to regular cars.
Consumers may have opportunities to receive a tax deduction for purchasing an alternative fuel vehicle, but this will vary by location and year.
Will Ethanol Use Affect the Price of Groceries?
This is a complex and difficult question to answer. In 2006 corn prices rose to historically high levels. Because corn is an important animal feed, this price increase could be passed to the consumer in the form of higher meat prices.
Increased ethanol production did factor into the increase in corn prices. But there were other factors, such as heavy demand from international markets. In 2006 the U.S. surpassed the 2 billion bushel export mark for only the fifth time in its history. The U.S. is the world’s largest corn producer, and other countries call on the U.S. grain supply when there are shortages of corn or other grains in their region.
High prices encourage farmers to increase their corn acreage so they can maximize their profit. In 2007, farmers responded to high corn prices by planting the largest U.S. corn crop since World War II. A 15 percent increase in corn acreage occurred in 2007 versus 2006. The additional 10 million acres planted with corn in the United States in 2007 reduced the supply of other crops and may have drawn some land out of the conservation reserve program (CRP).
What Do Ethanol Critics Say?
Three main areas of concern arise when people discuss the use of ethanol as an alternative fuel: economics, environment, and expectations.
From an economic standpoint, ethanol is not necessarily a cheaper alternative to gasoline and there are serious concerns about the impact higher grain prices will have on the U.S. economy. Also, ethanol is difficult and expensive to transport. Because ethanol is corrosive and absorbs water, it cannot be efficiently transported through pipelines.
There are environmental concerns about the ramifications of increased corn production. Corn relies heavily on petroleum-based crop inputs to maximize grain yield. Also, evidence exists that ethanol may increase volatile organic compound (VOC) emissions from automobiles.
Finally, ethanol is often oversold by groups with a vested interest in its success, and it may eventually suffer from unrealized expectations. The key point to remember is ethanol is just one small part of the renewable, alternative fuel solution and not the only answer.
What Is North Carolina’s Role in Ethanol Production?
Currently, North Carolina plays a small role in ethanol production. Because North Carolina imports large amounts of grain from the Midwest to feed livestock and poultry, corn-to-ethanol may not be the best ethanol production model to follow.
Ethanol production plants have been proposed in the state, and a few companies have acquired the necessary environmental permits to begin construction and operation. However, many analysts question the viability of building a large plant that will consume the state’s already limited grain resources.
There may be advantages to importing some feedstocks for ethanol. North Carolina would benefit from having local production plants that would allow local blending and distribution of ethanol.
Analysts anticipate that North Carolina’s role in ethanol production will increase as more appropriate feedstocks are identified. The key is identifying energy crops that can be competitively grown within the state.
As these new feedstocks are identified, the conversion process will need to be modified to facilitate the production of ethanol.
North Carolina State University researchers are currently working to make ethanol conversion from sweetpotatoes, sweet sorghum, trees, cotton stalks, switchgrass,
and duckweed a reality. Other potential feedstocks include residues from agriculture, food processing, pulp and paper processing, and from municipal wastes.
Can I Buy Ethanol in North Carolina?
Yes. In fact, many consumers across the state routinely purchase E10 blends—oxygenated gasoline formulations that reduce pollution. These blends are commonly found in urban areas to help reduce air pollution.
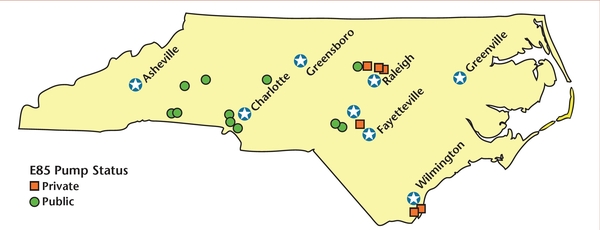
For higher ethanol blends, consumers must locate service stations that are properly equipped to dispense ethanol. North Carolina currently has 17 service stations either in-service or under construction offering E85. Twelve of these stations are open to the public; the remaining five are used to service government fleet vehicles (Figure 6). The
U.S. Department of Energy’s alternative fuel data center (AFDC) has a Web site that can be used to locate all E85-equipped service stations across the country. The E85 station locator can be found at on the U.S. Department of Energy website.
Is Ethanol THE Solution?
Ethanol is a promising alternative fuel that is renewable, better for the environment than petroleum-based fuels, and can be produced domestically. It also helps rural communities by providing jobs through the construction and operation of biorefineries and improving U.S. farm incomes. E85 may be best utilized in large cities and other highly congested locations where reduction in harmful emissions is critical. However, ethanol is not the only solution to rising energy costs. Ethanol is one part of an overall plan to help the U.S. achieve greater energy independence.
Other biofuels, such as methane captured from hog lagoons and biodiesel produced from vegetable oils, will play an important role in lowering energy costs and displacing petroleum consumption in the United States. Renewable energy sources, such as wind, solar, and biomass, will also provide signification contributions to the solution.
Finally, energy conservation is essential to reduce energy costs and help the United States achieve greater energy independence. Energy conservation may involve relatively minor lifestyle changes, such as using public transportation or adjusting the thermostat before leaving the house. It can also involve larger financial commitments like buying Energy Star rated appliances or a hybrid vehicle. Energy conservation is the best way to reduce demand and help stabilize prices.
References and Additional Resources
The following resources were used to produce this publication. Additional information about ethanol, biofuels, U.S. agriculture, and N.C. State University research programs can be found at the following Web sites:
Bio-Fuels
North Carolina State University
Ethanol
American Coalition for Ethanol
Renewable Energy
National Renewable Energy Laboratory
Energy Information Administration (EIA)
Flex Fuel Vehicles
U.S. Agriculture and Corn
Production
USDA National Agriculture Statistics Service
National Corn Grower’s Association
North Carolina agriculture statistics
Ethanol Economics
Did You Know?
In 2006, U.S. imports of oil dropped by 117 million barrels as a result of record production and use of ethanol.
This reduction in imports saved $11 billion from being sent to foreign countries for oil.
Using 7.5 billion barrels of ethanol will reduce demand for foreign oil by 2 billion barrels.
Publication date: Oct. 1, 2007
Reviewed/Revised: Sept. 27, 2024
AG-687
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.