Prepared by
M.S. Castillo, Associate Professor, Grassland Science Specialist Crop and Soil Sciences
At best, conserved forages can rarely match the nutritive value of fresh forage because some losses of highly digestible nutrients (sugar, protein, and fat) are unavoidable during conservation and storage.
High-moisture silage is ≤ 30% dry matter concentration.
Medium-moisture silage is 30% to 40% dry matter concentration.
Low-moisture silage (also called haylage, baleage, or wilted silage) is ~40% to 60% dry matter concentration.
Introduction
During periods of shortage of fresh forage caused by limited pasture growth or inadequate pasture conditions, forages can be conserved to feed livestock or fed as a supplement. Conserved forages can take the form of hay, haylage, or silage. Although several methods have been proven as efficient ways to store and preserve forages, it is important to remember that conserved forages can rarely match the nutritive value of fresh forage because some losses of sugar, protein, and fat are unavoidable during conservation and storage. Our goal in forage conservation is to focus on minimizing losses, which start immediately after cutting.
When selecting a conservation method, a producer should consider the suitability of the forage for a given method, storage capability, weather conditions, and the intended use of the conserved forage. The selected conservation technique should maximize nutrient conservation efficiency and minimize production costs.
What is Silage?
Silage is the final product when forage of sufficient moisture (> ~50%) is conserved and stored anaerobically (oxygen-free), under conditions that encourage fermentation of sugars to organic acids. The acidity generated by the organic acids (mainly lactic acid, but also acetic and propionic acids) and the lack of oxygen prevent the development of spoilage microorganisms. Three of the most critical factors for silage production are (1) rapid removal of air, (2) rapid production of lactic acid that results in a quick lowering of the pH (this is the result of adequate fermentation processes), and (3) rapid feedout once the silo is opened and exposed to air to avoid heating and spoilage.
Differences between Silage and Haylage
The main difference between silage and haylage is the initial dry matter (DM) concentration level at which the forage is clipped and packed to achieve optimum anaerobic and fermentation conditions. Three different moisture levels can be achieved: high-moisture silage (≤ 30% DM), medium-moisture silage (30% to 40% DM), and low-moisture (wilted) silage (40% to 60% DM). Low-moisture silage is referred to as haylage. When baled and wrapped, haylage is referred to as baleage. High-moisture silages are more prone to potential seepage losses (that is, effluent or leachate from the silo), undesirable secondary fermentation (resulting in butyric acid, which results in a rancid smell), and high dry matter losses (silo shrink). On the other hand, preservation as haylage depends more on achieving adequate packing (high density) to maintain anaerobic conditions. Achieving high density at packing is more difficult in drier forage. Nevertheless, high density is critical in haylage to maintain anaerobic conditions because microbes are less active and fermentation is lower in haylage than in higher moisture silage.
The Ensiling Process
Biology
Silage fermentation can be classified as either primary (desirable) or secondary (undesirable) (Pahlow et al., 2003). Primary fermentation is carried out by lactic acid–producing bacteria and is classified as homofermentative (the one product of fermentation is lactic acid) and heterofermentative (multiple products of fermentation are lactic and acetic acids and ethanol). Secondary fermentation is carried out mainly by enterobacteria (which produce lactic, acetic, succinic, and formic acids, and ethanol), clostridia (produce butyric acid), and yeasts (produce ethanol). Lactic acid production is preferred over the other fermentation products because it drives faster and lower pH drop (stronger acid) and limited silo shrink. Shrink occurs from plant and microbial respiration, fermentation, runoff, and loss of volatile organic compounds. If anaerobic and acidity conditions are not met, silage is more prone to shrinking during storage than is hay. Good fermentation should result in DM losses of less than 10%.
Phases of silage fermentation
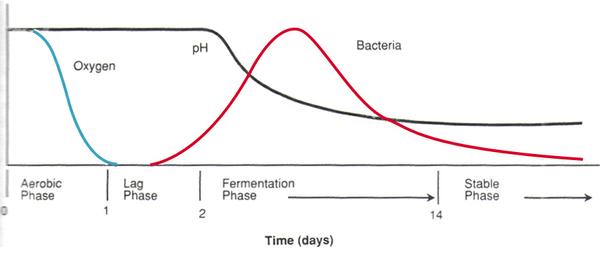
An overview of the four phases of the silage fermentation process (Collins and Owens, 2003) is shown in Figure 1. The phases are as follows:
- Aerobic: The aerobic phase usually lasts for approximately one day. During this period, plant cells and microbes will metabolize sugars and starch in the presence of oxygen, generating heat in the process. Silage temperature is elevated to about 90°F, and water may be lost (as seepage) because of respiration and compaction. If anaerobic conditions are not achieved quickly, high temperatures (>120°F) and prolonged heating will occur due to the growth of unwanted aerobic bacteria, yeast, and molds that compete with beneficial bacteria for substrate. Therefore, it is critical to ensure good compaction, proper moisture, and good sealing, all of which lead to a rapid transition to anaerobic conditions.
- Fermentation: Once anaerobic conditions are achieved, lactic acid bacteria and other anaerobes start to ferment sugars into lactic acid, mainly, and other organic acids to a lesser extent (such as acetic and propionic), which will drop the silage pH from about 6.0 to a range of 3.8 to 5. Alcohols such as ethanol will be generated too, but with no contribution to the acidification process. Rapid decrease in pH prevents breakdown of plant proteins and helps inhibit growth of spoilage microbes. Consequently, lactic acid production is preferred to ensure a low silo shrink. The fermentation phase usually lasts from one week to more than a month, depending on crop and ensiling conditions.
- Stable: As long as anaerobic conditions are maintained, silage can be stable for months and up to years. Under practical conditions, however, silage should be used within a year of its production. Slow entry of air through areas that were not properly sealed can slowly deteriorate material, so silos should be constantly checked and maintained to avoid any potential break of seal integrity.
- Feedout: Once a silo or bale is opened, it should be used as quickly as possible to avoid aerobic deterioration of the material. When oxygen becomes available in the ensiled material, yeasts metabolize the organic acids, which in turn cause the pH to increase, and restart the aerobic activity (such as growth of molds), causing greater silage spoilage. The design of a typical silo face should allow for the daily removal of approximately 6 inches of face material (for reference, each 6-inch daily removal is equivalent to one week of exposure to air). Silo opening should occur only after the fermentation phase has been completed (that is, after three to six weeks). The suggested approach is to wait approximately two to three months before opening a silo.
Management Practices for Making Better Silage
Crop Factors
An ideal crop to be ensiled should have an adequate level of sugars (measured as water-soluble carbohydrates, WSC) to be fermented, low buffering capacity (that is, resistance to changes in pH), and a stand with a dry matter concentration above 20% (McDonald et al., 1991). Corn is usually considered an ideal crop for ensiling because of its higher WSC compared to other forages and because dry matter concentration is usually about 30% to 35% when harvesting at milk stage. The concentration of WSC is critical for fermentation and varies among crops.
Table 1 shows minimum WSC concentration levels as a function of initial DM concentration and silage crops. Legumes have higher buffer capacity (resistance to allowing a drop in pH) and, therefore, require further wilting relative to other crops (35% to 45% DM) for adequate ensiling. In general, crop suitability for ensiling follows this order: corn, sorghum > ryegrass, orchardgrass, fescue, small grains > switchgrass, bermudagrass > legumes.
| Dry matter concentration (%) | Minimum initial WSC requirement (% of DM) [1] | ||
|---|---|---|---|
| Alfalfa | Grass | Corn | |
| 20 | 25 | 19 | 14 |
| 25 | 21 | 14 | 10 |
| 30 | 17 | 10 | 7 |
| 35 | 14 | 7 | 5 |
| 40 | 10 | 5 | 4 |
| 45 | 7 | 3 | — |
| 50 | 6 | 2 | — |
| Range of WSC at harvest (% of DM) | 4%–15% | 10%–20% | 8%–20% |
[1] Numbers in shaded boxes indicate insufficient WSC concentration for adequate fermentation. Notice the need for wilting alfalfa (and legumes) before ensiling. ↲
Harvest Maturity
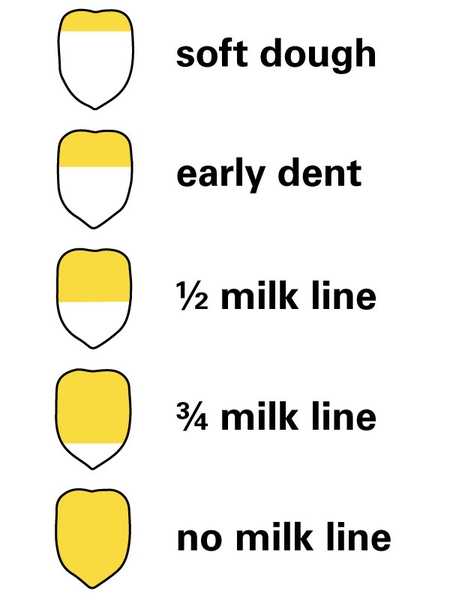
Table 2 presents harvest stages that optimize for yield, nutritive value, and fermentability of different crop and silo types. In the case of legumes, grasses, and cereals, maturity stage defines optimal harvest time and wilting is required in most cases to achieve the target DM concentration for ensiling. Corn, however, is different. Although examining the kernel milk line is always recommended (Figure 2), the decision of when to harvest should be made based on the DM concentration of the standing crop because it is ensiled directly afterward.
| Crop | Stage to harvest | ~Dry matter (DM) concentration at harvest (%) | Management suggestions for ensiling |
|---|---|---|---|
| Alfalfa | Bud to 10% flower [1] | 15–30 [1] | Wilt to 30%–50% DM [1] |
| Annual ryegrass, tall fescue, orchardgrass | Boot to heading [2] | 15–30 [2] | Wilt to 35%–50% DM [3] |
| Bermudagrass | First cut: Pre-head (12–15" tall); additional cuts: 4–5 weeks [2] | 18–30 [2] | Wilt to 40%–50% DM [4] |
| Corn | 1/4-2/3 milk line down the kernel [5] | 30–35 [5] | Direct cut [5]. Use 32%–40% and 33%–38% DM for bag and tower silos, respectively. [6] |
| Forage sorghum, sorghum-sudan, millet | Boot or soft dough [7, 8] | 30–35 [7] | Direct cut [7] |
| Small grains | Boot to soft dough [2] | 20–30 [2] | Wilt to 35%–45% DM |
| Switchgrass, gamagrass | Boot to heading [9,10] | 25–30 | Direct cut [9] |
[1] Albrecht, K. A., and K. A. Beauchemin. 2003. “Alfalfa and Other Perennial Legume Silage.” In Silage Science and Technology. No. 42, Agronomy. Madison, WI: ASA-CSSA-SSSA. ↲
[2] Adesogan, A. T., and Y. C. Newman. 2014. Silage harvesting, storage, and feeding. SS-AGR-177. University of Florida, IFAS Extension, Gainesville, FL. ↲
[3] Kunelius, T., and P. Boswall. Producing annual ryegrasses for pasture, silage, and seed. Agriculture and Agri-Food Canada. ↲
[4] Hersom, M., and W. E. Kunkle. 2011. Wilting bermudagrass improves forage silage quality and cattle performance. AN 145. University of Florida, IFAS Extension, Gainesville, FL. ↲
[5] Allen, M., J. G. Coors, and G. Roth. 2003. “Corn Silage.” In Silage Science and Technology. No. 42, Agronomy. Madison, WI: ASA-CSSA-SSSA. ↲
[6] Bagg, J., G. Stewart, and T. Wright. 2013. Harvesting corn silage at the right moisture. Order No. 13-051. Ontario Ministry of Agriculture and Food, Guelph, ONT. ↲
[7] Bean, B., and M. Marsalis. 2012. Corn and sorghum silage production considerations. High Plains Dairy Conference. Texas A&M AgriLife Extension, College Station, TX. ↲
[8] Lang, B. 2001. Sudan/sorghum: Forage management. Iowa State University Extension, Ames, IA. ↲
[9 ] Burns, J. C., D. S. Fisher, and K. R. Pond. 1993. Ensiling characteristics and utilization of switchgrass preserved as silage. Postharvest Biology and Technology 3:349-359. ↲
[10] Burns, J. C., and E. S. Leonard. 2013. Silages of native switchgrass and gamagrass: Fermentation characteristics, nutritive value, and quality. Tech. Bull. 332. NC Cooperative Extension, NC State University, Raleigh. ↲
Moisture
Moisture concentration affects the rate and extent of fermentation. Forages should not be ensiled with more than 70% moisture (or less than 30% DM concentration) due to potential seepage losses and growth of undesirable bacteria (such as clostridia), which results in undesirable fermentation. Wilting is needed in most cases when ensiling grasses and legumes.
As moisture decreases, less acidity is needed to inhibit undesirable bacteria that are more sensitive to low moisture than the desirable lactic acid bacteria. Low moisture is one of the factors that explain why wilting is beneficial for legumes and grasses. When wilting forages, adjust the mower-conditioner for a narrow swath, and then allow the swath to dry without further manipulation until the crop is chopped (Rotz, 1995). Research shows that this procedure minimizes field losses of the plant leaves, which are of higher nutritive value than stems. Table 1 includes recommended DM concentrations at harvest for different crops.
Particle Size
The optimal particle chop length is a balance between the particle size needed to achieve good compaction in the silo and the effective fiber requirements of ruminant livestock, especially lactating animals. The recommended theoretical length of cut (TLC) is 3/8 to 1/2 inch for unprocessed corn and legume silages, and 3/4 inch for kernel-processed corn silage (Muck and Kung, 2007). Sorghum silage should have a similar TLC to corn silage and grasses, and cereal silages should have a similar TLC to legume silages. Kernel processing is highly recommended when making corn silage to improve starch digestibility. Kernel processing should not be done, however, if whole plant DM concentration is less than 30% due to risk of increased seepage losses.
Packing Density
Attaining a high density in a silo is important because it determines the porosity at which air moves into the silo and subsequently the amount of spoilage that occurs during storage and feedout. Silage density is influenced by DM concentration, TLC, and packing intensity. Different types of silos require different packing strategies and target densities for appropriate ensiling. In general, a shorter TLC and processing will result in higher silage densities due to a decreased stiffness of the material. Silage density recommendations are provided in the Silo Type section. A general rule is to try to achieve a packing density of about 14 pounds per cubic foot (lb/ft3).
Sealing
Good sealing with plastic sheets and concrete barriers will keep the carbon dioxide in and prevent oxygen from entering the silo. Care must be taken to seal any holes with ultraviolet light–resistant tapes, especially in low-moisture silages where porosity is greater. Oxygen barrier film technology, compared to standard polyethylene films, has proven to further reduce DM losses and increase aerobic stability from the outer layers of silos.
Additives
Several types of additives can be used in silage making. Additives can help in every phase of silage making. Nevertheless, good harvesting practices are the main drivers of silage quality. In general, additives can be classified as stimulants or inhibitors of fermentation, and as nutrient sources (Kung et al., 2003). Specific effects of additives include the following:
- provide fermentable carbohydrates (Table 3)
- inhibit undesirable types of bacteria and promote desirable bacteria
- furnish additional acids (such as propionic acid) directly to decrease pH
- modify moisture (Table 4)
- extend aerobic stability during feedout (bunk life)
Table 4. Materials Used to Absorb Excessive Water in Silages (Green and Mueller, 1995)
|
Material |
Water absorbing capacity
(per lb of material applied) |
Amount of material needed to absorb 100 lb of water |
|---|---|---|
| Beet pulp | 2.9 lb | 34 lb |
| Ground alfalfa hay | 2.3 lb | 43 lb |
| Ground dry corn cobs | 2 lb | 50 lb |
| Ground ear corn | 1.3 lb | 77 lb |
| Ground shelled corn, wheat | 0.7 lb | 142 lb |
Inoculants, enzymes, and sugars can be considered stimulants of fermentation that promote rapid pH drop, produce more lactic acid, and reduce ammonia production (by preventing protein degradation) in most cases. These factors lead to a reduced silo shrink. Inoculants can be classified as homofermentative, heterofermentative, or containing a combination of both types of bacteria.
Inoculants containing heterofermentative bacteria, such as Lactobacillus buchneri, can extend bunk life during feedout by producing acetic acid, which is a powerful antifungal. A small increase in silo shrink may be observed in some instances, however, due to the transformation by L. buchneri of some lactic acid to acetic acid. This effect has been compensated for in most cases by combining L. buchneri with fast-growing homolactic bacteria. Fibrolytic enzymes are also added to most silage inoculants to help release extra sugars from fiber, thus stimulating fermentation, and to improve fiber digestibility. Other additives include inorganic and organic acids, which are considered inhibitors of fermentation. They are usually used in silages that have more than 70% moisture. Ammonia or urea are sometimes added to prevent fungal growth and supplement nitrogen in forages with low crude protein levels to improve forage nutritive value.
Silo Types
Piles and Bunkers
A pile silo consists of a layer of concrete or asphalt to protect from soil contamination and a polyethylene plastic sheet covering the silage (sometimes the wall as well). The bunker silo is a variant with two or three concrete wall sides (Figure 3). This silo type is the least expensive but also has a large surface exposed to the air. Consequently, adequate lining of the inside walls with plastic is needed to avoid outer layer forage damage, in addition to the plastic sheet for the top. Sealing is most critical to ensure good preservation and forage quality. Plastic films must be at least 5 millimeters thick and be ultraviolet light–resistant. Tires or gravel-filled bags must be used to keep plastic sheeting in place. Achieving at least 14 lb DM/ft3 is essential to minimize losses. The best silos can achieve densities of 20 lb DM/ft3. The forage crop should be packed to form progressive wedges and maintain a minimum slope of 1 (rise) to 3 (run) to avoid tractor rollover. The forage should be spread in a thin layer (no more than 6 inches deep) as much as possible to aid the compaction process.
Pressed Bags
Pressed bags, which are increasingly used, provide the advantage of flexibility in storage and movement of stored silage (Figure 4). The bagging machine regulates silage density in the bag, and achieving a smooth bag surface requires expertise. Pack as tightly as possible without creating an irregular surface (ripples), which creates air passages that can spoil much of the material being ensiled. Target density should be 14 lb DM/ft3 to achieve good results. Place the bag on a clean hard surface. This type of silo requires constant checking of each bag’s integrity so that punctures are quickly fixed and no air comes inside the silo.
Towers
Silage is filled by blowing the material through a pipe attached to the outside wall that ends in a distributor at the top of the tower silo. In concrete stave silos that unload from the top (Figure 5), the unloader blows the silage through doors located in the side of the silo and down a chute. The density in this type of silo is determined by the weight of the material on top and wall friction. Consequently, material at the bottom needs to be ensiled at a high DM (35% to 40%) to prevent effluent release. The upper surface of the silo is exposed to the air, and spoilage can occur up to 1 meter deep, which is discarded when emptying starts. The losses in DM during ensiling tend to be lower in tower silos compared to other types. Oxygen-limiting tower variants, primarily unloaded from the bottom, are available to limit spoilage even further.
Summary
Silage production is a bacterial-driven process in which crop, moisture, theoretical length of cut (TLC), and density interplay to generate the proper anaerobic and acidic conditions necessary for producing quality silage. When the proper conditions occur, they inhibit the growth of spoilage microorganisms, such as molds and yeasts. The main objectives are to obtain a silo with less than 10% shrink and silage that is high in nutritive value and stable for several days once exposed to air. Strategies for achieving these objectives under different conditions are outlined in this publication.
References
Adesogan, A. T., and Y. C. Newman. 2014. Silage harvesting, storage, and feeding. SS-AGR-177. University of Florida, IFAS Extension, Gainesville, FL.
Albrecht, K. A., and K. A. Beauchemin. 2003. “Alfalfa and Other Perennial Legume Silage.” In Silage Science and Technology. No. 42, Agronomy. Madison, WI: ASA-CSSA-SSSA.
Allen, M., J. G. Coors, and G. Roth. 2003. “Corn Silage.” In Silage Science and Technology. No. 42, Agronomy. Madison, WI: ASA-CSSA-SSSA.
Bagg, J., G. Stewart, and T. Wright. 2013. Harvesting corn silage at the right moisture. Order No. 13-051. Ontario Ministry of Agriculture and Food, Guelph, ONT.
Bean, B., and M. Marsalis. 2012. Corn and sorghum silage production considerations. High Plains Dairy Conference. Texas A&M AgriLife Extension, College Station, TX.
Burns, J. C., and E. S. Leonard. 2013. Silages of native switchgrass and gamagrass: Fermentation characteristics, nutritive value, and quality. Tech. Bull. 332. NC Cooperative Extension, NC State University, Raleigh.
Burns, J. C., D. S. Fisher, and K. R. Pond. 1993. Ensiling characteristics and utilization of switchgrass preserved as silage. Postharvest Biology and Technology 3:349-359.
Collins, M., and V. N. Owens. 2003. “Preservation of Forage as Hay and Silage.” In Forages: An Introduction to Grassland Agriculture, edited by R. F. Barnes, C.J. Nelson, K.J. Moore, and M. Collins, 443–471. Ames, IA: Blackwell Publishing.
Green, J. T., and P. J. Mueller. 1995. “Silage Production.” In Production and Utilization of Pastures and Forages in North Carolina, edited by D. S. Chamblee and J. T. Green. Tech. Bull. 305. Raleigh: NC Cooperative Extension, NC State University.
Hersom, M., and W. E. Kunkle. 2011. Wilting bermudagrass improves forage silage quality and cattle performance. AN 145. University of Florida, IFAS Extension, Gainesville, FL.
Israelsen, C., J. Barnhill, M. Pace, L. Greenhalgh, and J. Gale. 2009. “Harvesting Corn Silage by Plant Moisture,” AG/Farmland/2009-03pr. Logan, UT: Utah State University Cooperative Extension.
Kunelius, T., and P. Boswall. Producing annual ryegrasses for pasture, silage, and seed. Agriculture and Agri-Food Canada.
Kung, L., M. R. Stokes, and C. J. Lin. 2003. “Silage Additives.” In Silage Science and Technology, edited by D. R. Buxton, R. E. Muck, and J. H. Harrison, 305–360. Madison, WI: ASA-CSSA-SSSA Publishers.
Lang, B. 2001. Sudan/sorghum: Forage management. Iowa State University Extension, Ames, IA.
McDonald, P., N. Henderson, and S. Heron. 1991. The Biochemistry of Silage. Aberystwyth, UK: Chalcombe Publications.
Muck, R. E., and L. Kung. 2007. “Silage Production.” in Forages: The Science of Grassland Agriculture, edited by R. F. Barnes, C. J. Nelson, K. J. Moore, and M. Collins, 617–633. Ames, IA: Blackwell Publishing.
Pahlow, G., R. E. Muck, F. Driehuis, S. J. W. H. Oude Elferink, and S. F. Spoelstra. 2003. “Microbiology of Ensiling.” In Silage Science and Technology, edited by D. R. Buxton, R. E. Muck, and J. H. Harrison, 31–94. Madison, WI: ASA-CSSA-SSSA Publishers.
Pitt, R. E. 1990. “The Biology of Silage Preservation.” In Silage and Hay Preservation, 5–20. Ithaca, NY: National Resource Agriculture and Engineering Service.
Rotz, C. A. 1995. “Field Curing of Forages.” In Post-Harvest Physiology and Preservation of Forages, 39–65. Madison, WI: CSSA.
Acknowledgment
This publication is a revision of an earlier version. The authors would like to thank J.J. Romero and J.C. Burns for their earlier contributions.
Publication date: May 6, 2024
AG-812
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.