Requirements
Microorganisms
- Some fungi and bacteria produce lactic acid during fermentation.
- An example of a lactic acid producing bacteria is Lactobacillus.
- Other bacteria which produce lactic acid include:
- leuconostoc mesenteroides
- pediococcus cerevisiae
- streptococcus lactis
- Bifidobacterium bifidus
Lactic Acid Bacteria
- Lactic acid bacteria refers to a large group of beneficial bacteria with similar properties, and all produce lactic acid as an end product of the fermentation process.
- They are widespread in nature and can also be found in our digestive systems.
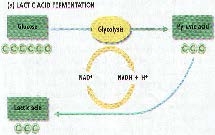
Homolactic fermentation
- The fermentation of 1 mole of glucose yields two moles of lactic acid
Heterolactic Fermentation
- The fermentation of 1 mole of glucose yields 1 mole each of lactic acid, ethanol, and carbon dioxide
Nisin
- Nisin was the first bacteriocin derived from the fermentation of a lactic-acid bacterium
- Approved by the FDA in April 1989 to prevent the growth of botulism spores in pasteurized process-cheese spreads.
- Does not inhibit Gram-negative organisms, yeasts, or fungi but does inhibit most Gram-positive organisms, including spore-formers such as Clostridia botulinum and heat-resistant spoilage organisms.
Homofermenter
- Enterococcus faecium
- Enterococcus faecalis
- Lactobacillus acidophilus
- Lactobacillus lactis
- Lactobacillus delbrueckii
- Lactobacillusleichmannii
- Lactobacillus salivarius
- Streptococcus bovis
- Streptococcus thermophilus
- Pediococcus acidilactici
- Pedicoccus damnosus
- Pediococcus pentocacus
Facultative homofermenter
- Lactobacillus bavaricus
- Lactobacillus casei
- Lactobacillus coryniformis
- Lactobacillus curvatus
- Lactobacillus plantarum
- Lactobacillus sake
Obligate heterofermenter
- Lactobacillus brevis
- Lactobacillus buchneri
- Lactobacillus cellobiosus
- Lactobacillus confusus
- Lactobacillus coprophilus
- Lactobacillus fermentatum
- Lactobacillus sanfrancisco
- Leuconostoc dextranicum
- Leuconostoc mesenteroides
- Leuconostoc paramesenteroides
Conditions for Lactic Acid Fermentation
- Addition of a sufficient amount of fermentable carbohydrates
- Reduced O2 during the fermentation process and storage of the fermented product.
- Rapid multiplication of the starter culture and sufficient production of lactic acid
Lactic Fermentation Products
- Western world: yogurt, sourdough bread, sauerkraut, cucumber pickles, and olives
- Fermented meats
- Middle East: pickled vegetables
- Korea: kimchi (fermented mixture of Chinese cabbage, radishes, red pepper, garlic, and ginger)
- Russia: kefir
- Egypt: laban rayab and laban zeer (fermented milks), kishk (fermented cereal and milk mixture)
- Nigeria: Nigeria: gari (fermented cassava)
- South Africa: magou (fermented maize porridge)
- Thailand: nham (fermented fresh pork)
- Philippines: balao balao (fermented rice and shrimp mixture)
Fermented Vegetables
- Sauerkraut
- Pickles
Fermentation
- Lactic acid fermentations are carried out under three basic types of conditions:
- dry salted
- brined
- non-salted
- Salting provides a suitable environment for lactic acid bacteria to grow, imparting an acid flavor to the vegetable.
How does salt preserve food?
- The chloride ion is a bacterial poison.
- Limits moisture availability.
- Oxygen solubility is reduced.
- Dehydrates protoplasm causing plasmolysis.
- Interfere with enzyme action.
- Generally, yeast, bacteria, and molds do not grow in saturated salt solution at 26.5% sodium chloride at room temperature.
Dry Salted
- Vegetable is treated with dry salt.
- The salt extracts the juice from the vegetable and creates the brine.
- As soon as the brine is formed, fermentation starts, and carbon dioxide bubbles appear.
- Fermentation takes one to four weeks, depending on the ambient temperature.
Sauerkraut
- Sauerkraut translates as acid cabbage. (Figure 5 and Figure 6).
- Leuconostoc mesenteroides.
- Lactobacillus plantarum.
- Shredded cabbage is placed in a jar, and salt is added.
- Mechanical pressure is applied to the cabbage to expel the juice, containing fermentable sugars and other nutrients suitable for microbial activity.
- The first micro-organisms to start acting are the gas-producing cocci (L. Mesenteroides). These microbes produce acids.
- When the acidity reaches 0.25 to 0.3% (calculated as lactic acid), these bacteria slow down and start to die off, although their enzymes continue to function.
- The activity initiated by the L. mesenteroides is continued by the lactobacilli (L. plantarum and L. Cucumeris) until an acidity level of ~1.5 to 2% is attained.
- The high salt concentration and low temperature inhibit these bacteria to some extent.
- Finally, L. pentoaceticus continues the fermentation, bringing the acidity to 2 to 2.5%, thus completing the fermentation.
Temperature Effect
- The optimum temperature for sauerkraut fermentation is around 21º C.
- A variation of just a few degrees from this temperature alters the microbial process's activity and affects the final product's quality
- Therefore, temperature control is one of the most critical factors in sauerkraut processing.
- A temperature of 18º to 22º C is most desirable for initiating fermentation since this is the optimum temperature range for the growth and metabolism of L. mesenteroides.
Effects of Salt
- Imparts firmness
- Inhibits putrefactive bacteria formation
- Withdraws water from the cabbage
- Added to a final concentration of 2.0 to 2.5%
Spoilage
- Aerobic soil micro-organisms break down the protein and produce undesirable flavor and texture changes
- Dark-colored sauerkraut (caused by spoilage organisms)
- Pink kraut is a spoilage problem. It is caused by a group of yeasts that produce an intense red pigment in the juice and on the surface of the cabbage
Brine Salted Fermented Vegetables
- For vegetables that inherently contain less moisture.
- A brine solution is prepared by dissolving salt in water (a 15 to 20% salt solution).
- Fermentation takes place well in a brine of about 20 salometers.
- The vegetable is immersed in the brine and allowed to ferment.
- The strong brine solution draws sugar and water out of the vegetable, which decreases the salt concentration.
- The salt concentration must not fall below 12%. Otherwise, conditions do not allow for fermentation. To achieve this, extra salt is added periodically to the brine mixture.
Pickles
- The washed cucumbers are placed in large tanks, and a salt brine (~15 to 20%) is added.
- The cucumbers are submerged in the brine, ensuring that no cucumbers float on the surface - this is essential to prevent spoilage.
- The strong brine draws the sugar and water out of the cucumbers, which reduces the solution's salinity.
- In order to maintain a salt solution so fermentation occurs, more salt must be added to the brine solution.
- If the concentration of salt falls below 12%, it will result in spoilage of the pickles through putrefaction and softening
- The color of the cucumber surface changes from bright green to a dark olive green as acids interact with the chlorophyll (Figure 7).
- The interior of the cucumber changes from white to a waxy translucent shade as air is forced out of the cells.
- The specific gravity of the cucumbers also increases due to the gradual absorption of salt, and they begin to sink in the brine rather than floating on the surface.
Publication date: Aug. 8, 2005
Reviewed/Revised: May 17, 2023
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.
This publication printed on: Feb. 28, 2026