What Is Basalt and Why Has It Been Offered for Application to Agricultural Soils?
Basalt is a dark-colored, dense, igneous rock formed from solidified lava. This silicate rock occurs in North Carolina in specific geological formations in Durham and Granville counties. Due to its hardness and durability, it is used in concrete and asphalt aggregates, concrete paving, railroad ballast, and other construction applications.
In the past few years, fine-ground basalt, also known as basalt dust or rock dust, became available on the North Carolina market as a soil amendment for application in agricultural fields. Using this material as a soil amendment could have some of the following advantages:
- Basalt captures carbon dioxide (CO2), and resulting carbon offsets could have value in the carbon credit trading market.
- Basalt raises the soil pH, so it could be a substitute for agricultural lime used to maintain adequate pH for crops.
- Basalt contains plant nutrients, including calcium, magnesium, manganese, iron, potassium, and copper. If released to the soil, these nutrients could help improve soil fertility.
Chemical Reaction of Basalt in Soils
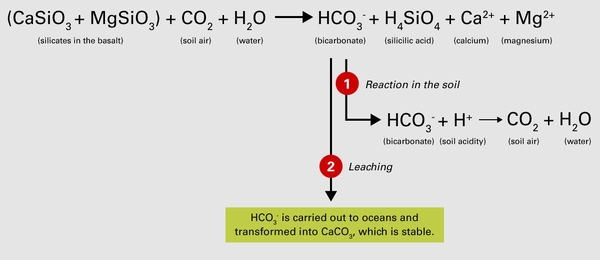
The role of basalt in the capture of CO2 and soil acidity neutralization is presented in Figure 1 and described here. During the weathering process, calcium and magnesium silicates (CaSiO3 and MgSiO3) of the basalt dust dissolve and react with CO2 and water from the soil atmosphere, forming bicarbonate (HCO3-). Other cations, including calcium (Ca2+) and magnesium (Mg2+), are released from the basalt and may be stored on soil particles, used by plants, or leached from the soil. Soluble silicic acid (H4SiO4) is also formed and easily leached from the soil. The formation of bicarbonate is the most noteworthy component in this reaction with regard to agronomic purposes. If bicarbonate stays in the soil (pathway 1), it neutralizes soil acidity (H+), raising the soil pH and converting back to CO2 and water. Bicarbonate is soluble, and, if leached out of the soil profile (pathway 2), it will be carried by groundwater to the ocean. There, other chemical reactions happen, and the carbon is stabilized as calcium carbonate (CaCO3). Both pathways may occur at the same time, but the extent of each depends on several physicochemical and environmental factors.
Basalt contains substances other than calcium and magnesium silicates, including aluminum compounds. If aluminum is released during the dissolution of basalt in acidic soils, it might contribute to lowering of the pH. This process is not considered in Figure 1.
For agronomic purposes, the most important characteristic of basalt to consider is its solubility. Basalt is a hard rock with limited dissolution. Consequently, the overall effectiveness of basalt in capturing CO2, increasing soil pH, and releasing nutrients is limited by its low solubility in water.
Incubation Study to Determine the Lime Equivalence
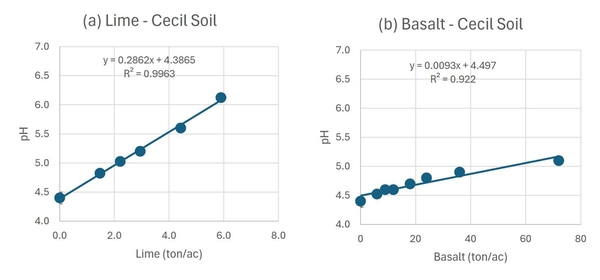
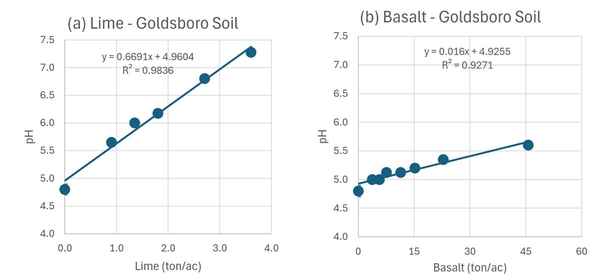
Using two representative soils from North Carolina, we conducted an incubation study to compare the effects of basalt and agricultural lime on soil pH. A Cecil soil with a pH of 4.4 and a lime requirement of 2.5 ton/acre to increase the soil pH to 6.0 was collected in Wake County. A Goldsboro soil with a pH of 4.8 and a lime requirement of 1.2 ton/acre to increase the soil pH to 6.0 was collected in Bertie County. We incubated 112 moist soil samples for 90 days with calcitic agricultural lime or fine-ground basalt. The Cecil soil received six rates of lime application (0, 1.5, 2.2, 3.0, 4.4, and 5.9 ton/acre) and eight rates of basalt application (0, 6, 9, 12, 18, 24, 36, and 72 ton/acre). The Goldsboro soil received six rates of lime application (0, 0.9, 1.4, 1.8, 2.7, and 3.6 ton/acre) and eight rates of basalt application (0, 3.8, 5.7, 7.6, 11.4, 15.2, 22.8, and 45.6 ton/acre). After 90 days, soil samples were analyzed at the North Carolina Department of Agriculture & Consumer Services Agronomic Division’s Soil Testing Laboratory.
Results: Lime Equivalence of Fine-Ground Basalt Rock
Agricultural lime significantly increased the pH of the Cecil soil. With the highest rate of lime (5.9 ton/acre), the soil pH increased from 4.4 to 6.1 in 90 days (Figure 2a). In contrast, the effect of basalt on soil pH was low even at the highest rate of application (72 ton/acre); at that rate, the pH increased from 4.4 to only 5.2 in the 90-day period (Figure 2b). For the Cecil soil, the calculated lime equivalence (LE) of the basalt was 30.8 tons/ton, meaning that a farmer would need to apply 30.8 tons of fine-ground basalt to achieve the same neutralization effect of 1.0 ton of agricultural lime.
Agricultural lime also significantly increased the pH of the Goldsboro soil. With the highest rate of lime applied, the pH increased from 4.8 to 7.4 in 90 days (Figure 3a). In contrast, the effect of basalt on pH was much lower. After 90 days of incubation at the highest basalt rate (45.6 ton/acre), the pH increased from 4.8 to 5.6 (Figure 3b). For the Goldsboro soil, the calculated LE was 41.8 tons/ton, meaning that a farmer would need to apply 41.8 tons of fine-ground basalt to achieve the same neutralization effect of 1.0 ton of agricultural lime.
The differences in the calculated LE of basalt between the Cecil and Goldsboro soils were due to the effect of the exchangeable acidity of each soil on the basalt dissolution. Cecil is a buffered soil with higher acidity, which helped to dissolve the basalt faster, reducing the calculated LE. Conversely, Goldsboro soil has lower acidity, and so the basalt was dissolved more slowly. As our results were from a limited 90-day incubation, we expect that when considering the long-term reaction of basalt, the LE of this amendment will be nearly the same in both soils.
Data on long-term incubations and diverse soil types are not available. Based on our limited dataset, however, we recommend an average LE of 35 tons/ton for basalt dust as a corrective of soil acidity. Farmers interested in applying this material could use Equation 1 to calculate the amount of basalt dust that would be equivalent to 1.0 ton of agricultural lime. (We are not endorsing use of this material; the equation is intended as a guide only.)
Equation 1
Basalt rate (ton/acre) = Lime rate (ton/acre) × 35
Effects of Fine-Ground Basalt Rock on Soil Nutrients and Other Soil Fertility Parameters
In our study, we also compared the effects of agricultural lime and fine-ground basalt on soil fertility. Use of lime increased the soil cation exchange capacity (CEC) and base saturation (BS), and it decreased the acidity (Ac) relative to the untreated soil or control; the same effects were observed with basalt dust but to a lesser extent (Table 1). The use of large rates of basalt increased both calcium (Ca) and magnesium (Mg), with the range of values within those recommended for agricultural soils (Table 1). Similarly, use of basalt increased copper (Cu), zinc (Zn), and manganese (Mn) indexes, with the range of values also within those recommended for agricultural soils (Table 2).
| Soil | Treatment | CEC (meq/100cc) | Ac (meq/100cc) | BS (%) | Ca (meq/100cc) | Mg (meq/100cc) | Ca (%) | Mg (%) |
|---|---|---|---|---|---|---|---|---|
| Cecil | Control | 4.25 | 3.45 | 19 | 0.4 | 0.3 | 10 | 6 |
| Lime (5.9 ton/acre) | 8.25* | 1.22* | 85* | 6.0* | 0.9* | 73* | 11* | |
| Basalt (72 ton/acre) | 6.52* | 2.65* | 59* | 1.9* | 1.8* | 30* | 28* | |
| Goldsboro | Control | 3.82 | 1.82 | 52 | 1.3 | 0.4 | 33 | 10 |
| Lime (3.6 ton/acre) | 6.45* | 0.12* | 98* | 5.3* | 0.8* | 82* | 12* | |
| Basalt (46 ton/acre) | 5.07* | 1.25* | 75* | 2.3* | 1.2* | 45* | 25* |
* Statistically different from the control treatment. ↲
| Soil | Treatment | P Index | K Index | S Index | Mn Index | Zn Index | Cu Index |
|---|---|---|---|---|---|---|---|
| Cecil | Control | 12 | 21 | 37 | 40 | 48 | 45 |
| Lime (5.9 ton/acre) | 16* | 17* | 43* | 30* | 33* | 35* | |
| Basalt (72 ton/acre) | 13 | 26* | 50* | 50* | 64* | 114* | |
| Goldsboro | Control | 105 | 65 | 45 | 27 | 31 | 38 |
| Lime (3.6 ton/acre) | 100* | 60* | 51* | 25* | 20* | 39 | |
| Basalt (46 ton/acre) | 97* | 63 | 48 | 44* | 35 | 93* |
* Statistically different from the control treatment. ↲
Based on the total concentration of Ca, Mg, Mn, and Cu in the basalt (data not shown) and the increase of these nutrients in the soil analysis after 90 days of incubation, the calculated average releases of Ca, Mg, Mn, and Cu were 16%, 7%, 11%, and 19%, respectively, during the incubation time.
The use of basalt increased both Ca and Mg in the soil, especially Mg (Table 1), thereby decreasing the Ca:Mg ratio. In the Cecil soil, the ratio decreased from 1.7:1 to 1:1. In the Goldsboro soil, the Ca:Mg ratio decreased from 3.3:1 to 1.8:1. Plants perform well in a wide range of Ca:Mg ratios, and no issues have been reported in the literature with Ca:Mg ratios between 1:1 and 8:1. However, only 7% of the Mg was released in 90 days; additional release of Mg would be expected beyond that window of time, which could further reduce the Ca:Mg ratios. Consequently, we recommend soil testing every year after basalt application to track the Ca:Mg ratios. We discourage repeated applications of heavy rates of basalt without monitoring Ca:Mg ratios.
High rates of basalt increased soil micronutrients, especially the Cu index (Table 2). Only 19% of Cu was released from the basalt in 90 days; we would expect additional release over time. Thus, we also recommend soil testing every year to track the levels of micronutrients after basalt application. We discourage repeated applications of heavy rates of basalt without monitoring micronutrients.
Publication date: June 10, 2024
AG-439-91
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.