Ground beetles (a.k.a.. carabid beetles or carabids) are a common, but often overlooked, beneficial insect in agricultural areas. Because they typically live in or on the soil and are usually active only at night, most people don’t notice them. However, they can be abundant and affect pest management. Some ground beetles are predators of other insects in the soil or upon plants, while others feed on weed seeds. Although scientists have studied ground beetles for years, because of their secretive habits there is still much to be learned about them. One of the first steps to appreciating the value of these beetles is to know which species are present and how to recognize them.
Abstract
This publication provides a quick guide to the various ground beetles that inhabit agricultural fields of the piedmont and eastern North Carolina. It offers Extension personnel, consultants, growers, scientists, and the general public an introduction to the identification and life histories of our most abundant ground beetles. We focus on adult beetles only, because they are much easier to collect and recognize than are larvae. Reference material is provided at the end of the article for those who are interested in learning more.
Ground Beetle Ecology and Habits
Ground beetles are predators that feed on insects, other invertebrates (such as snails), and seeds. They are specialized for many modes of life in and on the ground, as well as on plants, under bark, or as “miners” spending most of their time digging underground. Since agricultural settings are highly simplified environments, only a few of the many species of ground beetles are found there, though sometimes in great numbers. Agricultural settings tend to support ground beetles that are likely to feed on insect pests such as caterpillars and aphids, as well as weed seeds. Farmers have long been encouraged to look upon carabid beetles as naturally occurring pest control. Although it is difficult to say exactly how much pest control is provided by these beetles on individual farms, it is safe to say that they have a meaningful impact like other general predators such as ladybeetles and spiders. Current research is attempting to answer this question and learn what practices best enhance ground beetle activity in agriculture.
Conserving and Enhancing Ground Beetles
There are a number of strategies growers can employ that show promise for conserving beneficial ground beetles in crop fields. Ground beetles are generally vulnerable to deep or inversion tillage (e.g. moldboard plow) and benefit from reduced tillage regimes (Kromp 1999). Although adult ground beetles may be fast enough to escape from large disturbances like heavy plowing, their larvae and eggs are not mobile and are very susceptible to these disturbances. Increasing amounts of ground cover in crop fields benefit ground beetles, and cover crops can enhance beetle numbers (Shearin et al., 2008). However, cover crops are eventually turned under and destroyed and ground beetles need refuges where they can thrive and reproduce year round. Good refuges for ground beetles can be created in the borders of the field by planting a diverse mix of annuals and perennials (Menalled et al 2001). A similar strategy is to create these “beetle banks” within the crop field itself. Other strategies, such as intercropping and using organic fertilizers rather than mineral fertilizers, seem to promote ground beetles as well (Kromp 1999).
A specific strategy for weed seed eating ground beetles is to delay fall cover crop planting and fall tillage. This practice keeps weed seeds on the soil surface where they are more likely to be eaten by ground beetles (Gallandt et al., 2005).
Classification of Ground Beetles
Unfortunately for the average reader, the names that are used to describe the various types of ground beetles are based on taxonomic jargon. Because most of these beetles don’t have common names, we will also follow this convention. We have organized the various species of beetles by tribe. In addition to providing taxonomic organization, the tribe classification, to some degree, separates beetles based on their feeding habits. We have presented the basic hierarchical naming system used in insect classification (taxonomy and systematics):
- Order (e.g. Coleoptera, the beetles)
- Family (e.g. Carabidae, the ground beetles)
- Tribe (e.g. Cicindelini, the tiger beetles)
- Genus (e.g. Megacephala, the big-headed tiger beetles)
- Species (e.g. Megacephala carolina, the Pan-American big-headed tiger beetles)
- Genus (e.g. Megacephala, the big-headed tiger beetles)
- Tribe (e.g. Cicindelini, the tiger beetles)
- Family (e.g. Carabidae, the ground beetles)
The full species name of an insect includes the genus, species, the name of the person who described the species (the author), and the date the species was described, such as Megacephala carolina (Linnaeus, 1767). For the well-known authors, such as Linnaeus or Fabricius, the authors’ names are shortened to the first letter of the name. In the scientific literature (other than systematics), the date is usually omitted.
Handling Live Beetles
Only the largest of the ground beetles can manage a recognizable bite. However, many are capable of producing noxious chemical defenses in the form of foul odors, as in the Chlaeniini, or sometimes even acidic stinging spray as in the large Carabini. These chemical defenses are not used for attack but to dissuade their own predators (usually birds, or sometimes raccoons and other small mammals) in much the same manner as a skunk does.
After handling live beetles, it is always prudent to wash your hands before touching your eyes or any other sensitive skin. Otherwise, you may discover that the “Fiery Searchers” (Calosoma sp.) are aptly named!
Collections
This publication is based on collections made near Goldsboro, NC, in 2009 and 2010. The sites where we collected differ substantially in soil types, distance from water, and surrounding vegetation (see Forehand et al. 2006). Our collections were not geographically extensive throughout eastern North Carolina. However, Kirk (1972) and Thiele (1977) indicate that even relatively local, non-intensive collections will quickly reveal at least the dominant species in an area. Our collecting regime was intensive, conducted in three crops (corn, soybeans, hay) in nine fields, and was year-round, so we feel confident that we collected the vast majority (if not all) of the species that typically occur in these agricultural habitats.
| Tribe | General Feeding Habit (if known) | Species |
| Cicindelini | Predatory; stalk arthropod prey on the ground | Megacephala carolina (Linnaeus, 1767) |
| Megacephala virginica (Linnaeus, 1767) | ||
| Cicindela punctulata Olivier, 1790 | ||
| Harpalini | Herbivorous/Omnivorous: primarily feed on weed seeds but will also feed on other arthropods if needed. | Harpalus pensylvanicus (De Geer, 1774) |
| Harpalus caliginosus (Fabricius, 1775) | ||
| Acupalpus sp. | ||
| Amphasia sericea (T.W. Harris, 1828) | ||
| Anisodactylus merula (Germar, 1824) | ||
| Anisodactylus fervus LeConte, 1863 | ||
| Anisodactylus sanctaecrucis (Fabricius, 1798) | ||
| Anisodactylus laetus Dejean, 1829 | ||
| Anisodactylus sp. | ||
| Selenophorus palliatus (Fabricius, 1798) | ||
| Stenolophus infuscatus (Dejean, 1829) | ||
| Stenolophus lineola (Fabricius, 1775) | ||
| Stenolophus ochropezus (Say, 1823) | ||
| Stenolophus spretus Dejean, 1831 | ||
| Cratacanthus dubius (Palisot de Beauvois, 1811) | ||
| Chlaeniini | Mainly feed on arthropods, and other invertebrates, but also fungi | Chlaenius tomentosus (Say, 1823) |
| Chlaenius aestivus Say, 1823 | ||
| Chlaenius tricolor DeJean, 1826 | ||
| Chlaenius laticolis Say, 1823 | ||
| Pterostichini | Mainly arthropod and other invertebrate prey, especially caterpillars, and beetle eggs | Poecilus chalcites (Say, 1823) |
| Poecilus lucoblandus (Say, 1823) | ||
| Cyclotrachelus sigillatus (Say, 1823) | ||
| Pterostichus sculptus LeConte, 1852 | ||
| Carabini | Predatory; Mainly on caterpillars often found in agricultural settings | Calosoma sayi DeJean, 1826 |
| Carabus vinctus (Weber, 1801) | ||
| Platynini | Caterpillars, aphids, mealworms | Agonum pallipes (Fabricius, 1787) |
| Agonum punctiforme (Fabricius, 1823) | ||
| Agonum octopunctatum (Fabricius, 1798) | ||
| A. rigidulum (Casey, 1920) | ||
| Calathus opaculus LeConte, 1854 | ||
| Scaratini | Caterpillars, wire worms, other insects, and occasional plant matter | Scarites subterraneus Fabricius, 1775 |
| Galeritini | Caterpillars and grass seeds | Galerita bicolor Drury, 1773 |
| Liciniini | Caterpillars | Dicaelus elongatus Bonelli, 1813 |
| Cyclosomini | Small insects | Tetragonoderus intersectus (Germar, 1824) |
| Zabrini | Plant seeds, caterpillars | Amara cupreolata Putzeys, 1866 |
| Amara apricaria (Paykull, 1790) | ||
| Amara musculis (Say, 1823) | ||
| Bembidiini | Bembidion affine Say, 1823 | |
| Bembidion rapidum (LeConte, 1848) | ||
| Notiophilini | Notiophilus novemstriatus LeConte, 1848 | |
| Clivinini | Burrowing habit, tiny insects | Clivina bipustulata (Fabricius, 1801) |
The Beetle Species
Eleven tribes of carabid beetles were represented in pitfall trap collections taken from July through December 2009 in three corn fields, three soybean fields, and three hay fields near Goldsboro. Thirty-one species representing twenty genera and eleven tribes of carabids were identified from these collections (Table 1). Of these, nine genera and eleven species from six tribes made up approximately 99 percent of the species assemblage. Megacephala carolina alone accounted for 55.4 percent of the beetles collected, followed by Harpalus pensylvanicus (26.5 percent); Anisodactylus spp. (6.9 percent—note that Anisodactylus species are difficult even for experts to distinguish, and at least five species are represented in this collection); Poecilus chalcites (3.5 percent); Calosoma sayi (3.1 percent); Agonum punctiforme (1.7 percent); Cyclotrachelus sigillatus (1.3 percent); Cicindella punctulata (0.7 percent); and Chlaenius tomentosus (0.7 percent). For practical purposes, beetles collected with a frequency of 0.5 percent or more were considered common and are both described and illustrated below. Beetles with a collection frequency between 0.5 and 0.1 percent were considered uncommon and are only illustrated in Figure 14.
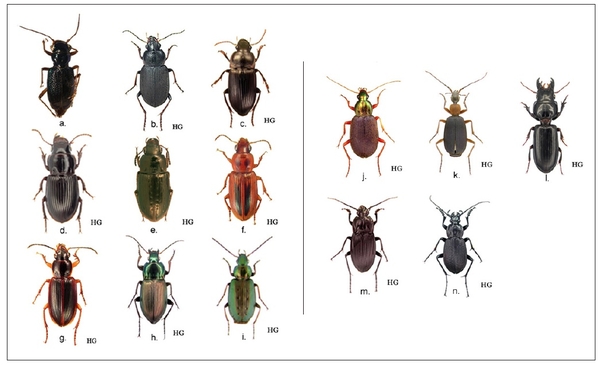
Figure 14. Occasionally encountered carabid beetles from eastern North Carolina agricultural fields. a. Cicindelini, Megacephala virginica. b- g. Harpalini, Amphasia sericea; Harpalus caliginosis; Cratacanthus dubious; Selenophorus palliatus (Photo by Igor Sokolov, LSU); Stenolophus lineola; S. ochropezus. h. Pterostichini, Poecilus lucublandus. i. Platynini, Agonum octopunctatum. j. Chlaeniini, Chlaenius tricolor. k. Galeritini, Galerita sp. l. Scaritini, Scarites subterraneum. m. Licinini, Dicaelus elongatus. n. Carabini, Carabus vinctus. Photos marked “HG” by Henri Goulet Agriculture and Agri-Food Canada. Images are not to scale.
Those with a collection frequency less than 0.1 percent were considered rare and are not illustrated.
Seasonality and Abundance
The life cycles of different types of carabid beetles vary, and so collections of adult beetles will vary with the seasons. In our collections, Cicindelini, Carabini, Chlaeniini, and Pterostichini were most abundant in the summer months, but were still found in smaller numbers in the fall. By late fall (December) they were rarely encountered. Harpalini were most abundant in the fall, but were found in much smaller numbers in the summer and late fall. Platynini never reached any appreciable numbers until late fall (December) when they became the dominant carabid trapped, though in much smaller numbers than carabids dominant in summer. A small number of Bembidiini species were found in a collection made in February only. An impressive number (>3000) of Amara cupreolata (tribe Zabrini Fig. 12m.) were found in one particular field in May, likely the result of a locally high density of annual bluegrass that was allowed to mature in that particular field.
Description of the Commonly Collected Ground Beetles
Ground beetles all share a few common features. They always have threadlike, long antennae that do not come to a club or branch (Figure 1). They have five tarsal (“foot”) segments on each leg (Figure 1), and an expanded hind trochanter (”hip joint”), which looks a bit like a jelly bean, lying next to the femur of the hind leg (Figure 2). They are all fast-running hunters, seed feeders, or both. The majority of carabid species are nocturnal, preferring to hide under rocks, logs, or leaf-litter during daylight hours. Like all beetles, the front wings of ground beetles are modified into distinctive protective shell-like coverings called elytra that protect the hind wings. Most carabid larvae build burrows in which they live. The depth of these burrows appears to be specific to each group. Predatory larvae attack passing insect prey, which they drag into their burrows. Seed-feeding larvae may line the walls of their burrows with stores of seeds.
About the Images
In a perfect world, field-caught specimens would look just like the pictures in this publication and make everyone’s job of identifying things a breeze. In the real world, however, specimens are seldom identical to their pictures and often require specific equipment for viewing. A good hand lens is indispensible, but the most important aspect of viewing the coloration of beetles is lighting. While the tiger beetles are often very easy to discern, some of the coloration on Chlaeniini and other groups is much more subtle and requires strong directed light, such as that provided by a dissecting-scope illuminator. Even with the appropriate lighting, shifting angles often produce a range of coloration. Also, coloration naturally varies even in the same species, and sometimes dead specimens look a little less vivid than live ones, which is why taxonomists try not to rely entirely on colors for identifications. Lines beside the images in Figures 4 to 13 represent actual size, when the page size in this article is 8.5 by 11 inches.
Tribe Cicindelini (Tiger beetles)
The adults of this group are easily recognized by their extremely long, almost spidery legs and massive jaws (Figure 3, Figure 4, Figure 5). The Cicindelini are the one tribe of carabids in which the labrum (top lip) is wider than the antennal insertions (Figure 3). Most tiger beetles are active in either the spring and fall, or the summer. Summer active species dominate in the south, while spring/fall active species are more common in the north (Pearson et al. 2006). Summer active adults die off at the end of the year, but their larvae overwinter in burrows that are deep enough to have moderate temperatures. Tiger beetles are thought to be valuable indicators of ecosystem health due to their easy identification and collection, as well as their specificity to an environ (Pearson and Cassola 1992).
Megacephala carolina (Pan-American big-headed tiger beetle, Carolina tiger beetle) (Figure 4) These beetles are predatory, nocturnal, often gregarious and will scatter from the light of a flashlight. These were by far the most abundant beetles in our collections.
Identification: They are easily recognized by their unique purple and green metallic surface, and yellow comma-shaped markings on the elytra (circle). This beetle is relatively large, usually near 20mm (0.79 inches).
Cicindela punctulata (punctured tiger beetle, sidewalk tiger beetle) (Figure 5) is a widely distributed upland beetle often found in agricultural settings. It is sometimes so common that it is believed to have developed some pesticide resistance (Larrochelle and Lariviere 2003). It has a 1- to 2-year life cycle.
Identification: Dark shining olive or tar-colored dorsally, with slight white markings on the lateral edges of the elytra, and metallic blue ventrally. This beetle is about 13mm (0.51 inches) long.
Tribe Harpalini
Harpaline beetles are recognized by having one seta (hair) above each compound eye, a mostly dark brown or black dorsal color, and all lack an interruption in the lateral edge of the elytra (see tribe Pterostichini for description of this feature). Species are often difficult even for experts to discern.
Harpalus pensylvanicus (Figure 6) is common in croplands and widely distributed throughout the contiguous U.S. and Canada. This beetle has been observed feeding on seeds of wheat, Timothy grass, and prairie grass. Gut content studies have found ragweed pollen as well as ants and mites (Kirk 1974). Because of their preference for seeds and because they are active in the fall months when summer annual weeds have shed their seeds, Harpalus is an important weed seed predator. Harpalus species have been shown to consume 90% of the seeds of certain weed species (Zhang, 1993). Kirk (1971) lists this as the second most prevalent species in North Dakota agricultural fields. There appears to be a preference for cultivated land, and aggregation in weedy grasses (foxtail grass) where the planter missed a row in corn fields (Kirk 1974). Larvae may provision burrows with grass seeds pasted to the sides of the tunnel-like tiles and pack such seeds around the opening (Kirk 1971).
Identification: Light tan legs and a somewhat shining, tar colored body. It is 13 to 15.5mm (0.59 inches) in length.
Anisodactylus fervus, Anisodactylus merula and Anisodactylus sp. (Figure 7) are a prevalent component of our collections in early to mid summer and are nearly replaced by H. pensylvanicus in the fall. It is omnivorous, feeding on seeds, caterpillars, and in laboratory on mealworms (Larochelle and Lariviere 2003). Laboratory experiments have shown that seed predation by these beetles can reduce weed pressure from redroot pigweed (Amaranthus retroflexus) and lambsquarters (Chenopodium album) (Kromp 1999). However, it is unclear what their effect is in agricultural fields because they are prevalent before weeds shed their seeds in the fall. This nocturnal, gregarious beetle, according to Larochelle and Lariviere (2003) favors human activities and is frequently found at roadsides, golf courses, and other disturbed areas.
Identification: Dark, black to rusty brown, and translucent at the posterior sides of the pronotum. In many, the protibial apical spur (circle) is trifid (trident-like). These beetles are about 8 to 13mm (0.51 inches) in length.
Tribe Pterostichini
These beetles are recognized by having two setae above each eye and an interruption in the lateral to apical edge of the elytra (circle).
Poecilus chalcites (Figure 8) was found in many of our pitfall samples. It is a common predator on a number of crop pests including western corn rootworm, corn earworm, black cutworm, and armyworm. This species is recorded from 35 states east of the Rocky Mountains and eastern Canada. It is often the most abundant ground beetle during most of the growing season in Illinois farmland (Lundgren et al. 2005). Lundgren et al. (2005) report that P. chalcites requires a diapause before becoming sexually mature.
Identification: This beetle has a distinctive smooth, bronze-green sheen. The green color will not be as distinctive as in Figure 8, except under special microscope lighting. It is about 10 to 12mm (0.47 inches) in length.
Cyclotrachelus sigillatus (Figure 9) found in the late fall is black and shining.
Identification: The pronotum is almost square and as wide as the elytra. The elytra do not open, rendering this beetle incapable of flight. They are 13 to 18mm (0.64 inches) in length.
Tribe Carabini
These relatively large beetles are often called “hunters” or “searchers” and famously prefer caterpillars. They have relatively large jaws and are often brightly marked or colored. They are capable of spraying noxious acidic defensive secretions.
Calosoma sayi (Figure 10) is among the largest beetles in our collections. Often called “fiery searcher” due to the acids it will spray on your hands if handled.
Identification: The characteristic elytral puncture-markings can be quite variable in color from bronze-red to blue-green. This is the largest carabid in our trap collections, often over 25mm (1 inch) in length.
Tribe Zabrini
Only two genera are contained in the Zabrini, and only the genus Amara is found in our area. The Amara are recognized by having characteristics of the Pterostichini: two setae above each compound eye and an interrupted lateral elytral edge. They differ from the Pterostichini in being oval in shape and lacking any punctures on the dorsal surface of the elytra. Amara also have many setae on the second segment of the labial palpi where many Pterostichini have only two (however this character alone will not suffice in separating the tribes).
Amara cupreolata (Sun beetles) (Figure 11) is common in our collections in May and early June. It can often be seen running about vigorously on bright sunny days. It is a known seed feeder preferring grasses.
Identification: Oval, shining, brownish-bronze colored body with dark legs. They are 6.6 to 9mm (0.35 inches) in length.
Tribe Chlaeniini
These velvety beetles are often metallic and brightly colored. They have a single seta (hair) above each eye like the Harpalini), but they also have interruption in the lateral edge of elytra like the Pterostichini and Zabrini. The Chlaeniini are famously foul-smelling.
Chlaenius tomentosus (Figure 12) is found in rural areas and in cities. It seems to prefer cultivated land, old fields, and vacant lots. This beetle emits a musky or smoky odor when handled.
Identification: It is a shining bronze-green beetle, covered in fine hair, like velvet on the elytra. It is 13 to 15mm (0.59 inches) in length.
Tribe Platynini
These mostly black, shining beetles have two setae above the eye (like the Pterostichini and Zabrini) and never have the lateral edge of the elytra interrupted.
Agonum punctiforme (Figure 13) was only apparent in our collections in the late fall and in warm winter weather.
Identification: It is a shining black beetle with a well rounded pronotum with two distinctive rounded dimples basally. The elytra are slightly less black than the head and pronotum and can reflect bronze green under strong lights. There are 3 dorsal punctures on the elytra in the third interval. It is 7 to 9mm (0.35 inches) in length.
For Further Information
References Cited
Ciegler, J. C. 2000. Ground beetles and Wrinkled Bark Beetles of South Carolina (Coleoptera: Geadephaga: Carabidae and Rhysodidae). Biota of South Carolina. Vol. 1. Clemson University, Clemson, S. C.
Forehand, L.M., D.B. Orr, and H.M. Linker. 2006. Evaluation of a Commercially Available Beneficial Insect Habitat for Management of Lepidoptera Pests in Organic Tomato Production. J. Econ. Entomol. 99: 641-647. ↵
Gallandt, E. R., Molloy, T., Lynch, R. P., and Drummond, F. A. 2005. Effect of cover-cropping systems on invertebrate seed predation. Weed Sceince 53:69-76. ↵
Kirk, V. M. 1971a. Ground beetles of cropland in South Dakota. Ann. Entomol. Soc. Am. 64: 238-41. ↵
Kirk, V. M. 1974. Biology of a Ground Beetle, Harpalus pensylvanicus. Ann. Entomol. Soc. Am. 66(3): 513-518. ↵
Kromp, B. 1999. Carabid beetles in sustainable agriculture: a review of pest control efficacy, cultivation impacts and enhancement. Agriculture, Ecosystems and Environment 74: 187-228. ↵
Larochelle, A. and M.C. Larivière. 2003. A Natural History of the Ground-Beetles (Coleoptera: Carabidae) of America north of Mexico. Pensoft Sofia, Bulgaria. ↵
Lundgren J. G., J. J. Duan, M. S. Paradise and R. N. Wiedenmann 2005. Rearing Protocol and Life History traits for Poecilus chalcites (Coleoptera: Carabidae) in the Laboratory. J. Entomol. Sci. 40(2): 126-135. ↵
Menalled, F. D., J. C. Lee, and D. A. Landis. 2001. Herbaceous filter strips in agroecosystems: Implications for ground beetle (Coleoptera : Carabidae) conservation and invertebrate weed seed predation. Great Lakes Entomologist 38(2): 472-483. ↵
Pearson, D. L., F. Cassola. 1992. World-Wide Species Richness Patterns of Tiger Beetles (Coleoptera: Cicindelidae): Indicator Taxon for Biodiversity and Conservation Studies. Conservation biology 6(3): 376-91. ↵
Pearson, D. L., C. B. Knisley, and C.J. Kazilek. 2006. A Field Guide to the Tiger Beetles of the United States and Canada. Oxford University Press. ↵
Shearin, A. F., Reberg-Horton, S. C. , and Gallandt, E. R. 2008. Cover crop effects on the activity-density of the weed seed predator Harpalus rufipes (Coleoptera: Carabidae). Weed Science 56(3):442-450. ↵
Thiele, H. U. 1977. Carabid Beetles in Their Environments. Zoophysiology and Ecology 10. Springer-Verlag. Berlin Heidelberg New York. ↵
Web Resources
Ground Beetles of Canada Image Library
Image Gallery “Carabidae of the World”
Books of Interest
Acorn, J. H. 2001. The Tiger Beetles of Alberta. Alberta Insects Series. The University of Alberta Press. Edmonton, Alberta. 120pp.
Ball, G. E., and Y. Bousquet 2000. Carabidae. In Arnett, R. H. Jr. (Editor), and M. C. Thomas (Editor) American Beetles, Volume I. Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia CRC Press. 464pp.
Ciegler, J. C. 2000. Ground beetles and Wrinkled Bark Beetles of South Carolina (Coleoptera: Geadephaga: Carabidae and Rhysodidae). Biota of South Carolina. Vol. 1. Clemson University, Clemson, S. C. 149pp.
Pearson, D. L., C. B. Knisley, and C.J. Kazilek. 2006. A Field Guide to the Tiger Beetles of the United States and Canada. Oxford University Press. 227pp.
White, R. E. 1983. A Field Guide to the Beetles of North America. The Peterson Field Guide Series. Houghton Mifflin Company. New York, NY. 368pp.
Prepared by
Geoff Balme, Department of Entomology, NC State University
David Orr, Department of Entomology, NC State University
Aaron Fox, Department of Crop Science, NC State University
11-CALS-2288
AG-735-01
2/11—VB/KEL
Publication date: Feb. 1, 2011
Reviewed/Revised: Aug. 23, 2023
AG-735-01
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.