Recall of Food Under FDA Jurisdiction
Recalls are an important tool to protect our nation's food supply chain and ensure the health and safety of consumers. Since 2013, the United States has seen a fluctuation in the number of FDA-driven food recalls. This could be due to multiple factors such as more accessible laboratory services, an increased awareness of safety measures becoming more proactive, rather than reactive since the Food and Drug Administration's (FDA) implementation of the Food Safety Modernization Act. With more than 1550 products recalled in the United States in 2023, the odds of recalls affecting your operation are a real risk1.
When is a Written Recall Plan Required?
Companies across multiple food manufacturing industries are required to have a written recall plan. Regardless of regulatory requirements or size, it is advisable for all food manufacturers to develop a recall plan. Starting to develop a recall plan can begin as simply as ensuring the company can track their ingredients one step back to the supplier and one step forward through their distribution routes.
- When a Food Safety Plan identifies a hazard requiring a Preventive Control
- Companies manufacturing Acidified foods
- Companies subject to Juice HACCP regulations
- Companies subject to Seafood HACCP regulations
- Companies manufacturing thermally processed low acid products
- As required by by third-party certifications (e.g. SQF, BRC, etc.)
Classes of Food Recalls
The numerical designations (i.e., I, II, or III) are assigned by the FDA to each product recall to indicate the relative degree of the health hazard presented by the product being recalled. Class I recalls are the most dangerous and are tracked through the FDA's Reportable Food Registry due to their high-risk to consumers.
| Class I | A situation in which there is a reasonable probability that the use of, or exposure to, a violative product will cause serious adverse health consequences or death. | An RTE product testing positive with a pathogenic microorganism. Ex: Salmonella spp. or Listeria monocytogenes Allergen adulterants or labeling problems (Exemption: Wheat) |
|---|---|---|
| Class II | A situation in which use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or the probability of serious health consequences is remote. | A product with a physical contaminant. Ex: Stones in produce or broken pieces of a gasket |
| Class III | A situation in which use of, or exposure to, a violative product is not likely to cause illness or injury. | Incorrect weight or volume Product that may have been manufactured under insanitary conditions |
| Market Withdraw | When a product has a minor violation that would not be subject to regulatory action | A product labeled as red and white chocolate candies but only contained red candies. |
| Stock Recovery | A company’s removal or correction of a product that has not been marketed or that has not left the direct control of the company. | Product located on premises owned by, or under control of the company. No affected products released for sale. |
When may the FDA initiate a mandatory recall?
Regulators will urge companies to initiate voluntary recalls of their products during a recall event. Historically, almost all companies have complied with these State of Federal requests for recall, but the FDA does retain the right to mandate the recall if a company doesn't comply in a timely fashion2,3.
Other times in which the FDA may mandate a recall:
- When exposure or consumption of a product will cause serious adverse health consequences or death to humans or animals (SAHCODHA risk)
- Examples of food products that represent a SAHCODHA risk:
- Peanut butter, alfalfa sprouts, and deli products found to be contaminated with Salmonella spp.
- Under-processed canned chili that contained Clostridium botulinum toxin
- Smoked salmon and pumpkin seeds found to be contaminated with Listeria monocytogenes
- Products containing undeclared allergens (e.g., milk, peanuts, or eggs)
- Baby food that posed a choking hazard
- Examples of food products that represent a SAHCODHA risk:
- As a response to significant food safety observations made during regulatory inspections
- Results from sample analyses, which may include those for raw materials, finished food products or from the processing environment
- Epidemiological data (e.g., food borne outbreak data directly related to the food product)
- If the food product serves a vulnerable population (e.g., infants, toddlers, the elderly, pregnant women, medically compromised individuals)
- Consumer and trade complaints
- If the responsible party has failed to initiate a voluntary recall2
Reportable Food Registry (RFR)
This FDA database houses information pertaining to reportable foods and Class I recalls. A “reportable food” is defined as “an article of food (other than infant formula) for which there is a reasonable probability that the use of, or exposure to, such article of food will cause serious adverse health consequences or death to humans or animals (SAHCODHA risk).
As soon as practical after discovering a “reportable food” — but in no case more than 24 hours later — a responsible party must submit a report to the FDA through the electronic RFR. A “responsible party” is a person who submits to the FDA the registration for the facility where the article of food at issue is manufactured, processed, packed or held. The report to the Food Registry must include: a description of the food, the quantity, the extent and nature of the adulteration, whether the adulteration might have originated with the responsible party, and the results of the required investigation, if known. The law states that submission of this information is not an admission that the food is adulterated or caused an injury.
Preparing Your Recall Team
Companies should always be recall-ready. Expediting recall activities is vital to an effective and efficient recall event. To begin, the recall plan must be a written recall plan. This document provides all information needed to successfully prepare for, conduct, evaluate, and terminate a recall event. The recall plan should be evaluated, updated and training conducted on the most up-to-date information on a routine schedule.
Assemble Your Recall Team
Gather a cross-functional team and assign specific responsibilities in the case of a recall. Ensure to include a few alternate team members in case of absence or position vacancy.
Roles to Consider Including on your Recall Team
Recall coordinator
Operations manager
Publicity & public relations
Sales & marketing
Logistics & receiving
Quality assurance
Accountants
Scientific advisor
Attorney
Administrative support
FDA recall coordinator
State recall coordinator
Responsibilities to consider are:
- Who will initiate the recall?
- Who will communicate with regulators?
- Who will notify direct customers, consignees, and distributors?
- Who will be responsible for notifying the public, when appropriate?
- Who will gather documents, compile data and make reports?
- Who will be responsible for conducting effectiveness checks?
- Who will be responsible for securing effected inventory and managing its disposal?
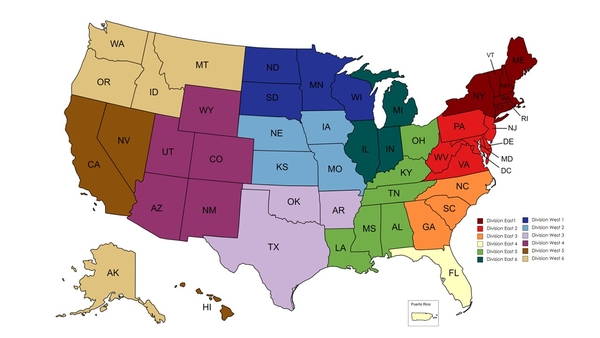
Contact lists should be developed for contacting regulators, consumers, and the public. The FDA's Office of Regulatory Affairs has a team of Recall Coordinators, responsible for assisting in recalls in their Divisions6, as seen in Figure 1. Other contacts to include would be: distributors and retailers of your product, insurance agents, and local media.
Recall letters, media publications, press releases and other communication streams should be drafted and ready to be used in the event of a recall. Companies can go back to their Food Safety Plan's Hazard Analysis and prepare recall letters or other communications for the hazards identified. An annual review and update of contacts and personnel will help keep the communication templates accurate. It can be helpful to provide a company contact who is responsible for customer questions. All communications should be customized to fit the recall event and contamination of concern. Reviewing the Hazard Analysis section of your company's Food Safety Plan can help assess other hazards to be prepared for in the event of a recall. The FDA has created mock recall letter templates for Listeria monocytogenes and Salmonella contamination, as seen in Figure 2.5 and Figure 3.7. Mississippi State University Extension has put together a resource for Developing a Crisis Communication Plan that can be helpful for your Team.
Mock Recalls: Verifying your Recall Plan is Effective & Your Team is Prepared
Mock recalls should be conducted at regular intervals. Deciding on their frequency depends on the risk of the food being involved in a recall, current evidence of an elevated risk, regulatory and auditing requirements, staff turnover, among others. As many Recall Team members should participate in the mock recall and they should perform their recall responsibilities in full during the mock scenario.
Management can test members' knowledge on:
- When a recall would need to be initiated
- Who to contact during a recall
- How to contact technical help
- Product traceability, can employees track products one-step forward and one-step back
- Record gathering, recall documentation and accurate template utilization
Handling an Active Recall
Appropriate and thorough communication is key in conducting an efficient and effective food recall. Communication to regulators and consumers allows recalled items to be swiftly removed from commerce and properly destroyed or returned to the recalling company. At times, your company may be contacted by a regulator because of a possible implication in an outbreak or recall event. The International Fresh Produce Association has created a helpful document that can help guide conversation when contacted by an agency.
Your State Recall Coordinator should be contacted as soon as a recall event has been initiated4. This allows them to provide insight into the recall process but also put forth efforts to assist the company and consumers. The FDA Recall Coordinator for your region should also be contacted, especially with any interstate distribution of your product.
When you contact distributors, retailers and consumers be sure to adequately inform them about the recalled product and any actions they should take. All written communications should be flagged in large, bold print "URGENT: FOOD RECALL". Any distribution envelopes should be similarly flagged8.
FSPCA has created a template that you can customize to your company’s needs.
Information to gather during a recall event:
-
Recalling company's name, address, recall contacts and scope of the operation (Manufacturer, packing, holding, warehousing, or distribution)
-
Recall initiation date
- Product name (both brand and generic names)
- Product image
- Labels of all affected product (2 copies of each label to go to your Regulatory partners)
- Description of product:
- Product size or volume
- Type of packaging (box, flexible plastic, glass, bulk)
- Expected shelf life
- Storage and distribution conditions
- Lot Codes or unit numbers
- Reason for the recall
- Explain how the problem occurred and the date(s) it occurred.
- Explain how the problem was discovered and the date it was discovered.
- Out-of-specification testing results
- Explain in detail how the product is violative
- If the recall is due to the presence of a foreign object, describe the foreign object’s size, composition, hardness, and sharpness
- If the recall is due to a chemical contaminant, explain the level of contaminant in the product and provide the Safety Data Sheet for the contaminant
- If the recall is due to the presence of a pathogen, provide the test results if requested.
- If the recall is due to a label issue (e.g., a missing or inaccurate ingredient list), provide and identify the correct and incorrect label, description, and formulation.
- Detailed information on complaints associated with the product/problem, such as reports of adverse events
- Dates and description of complaints including injury or illness
- Amount of affected product the company is still retaining and how it is being quarantined
- Distribution information with contact information
- Consignees & direct consumers
- Distributors and wholesalers
- Downstream manufacturers
- Retail outlets
- Foreign exportation (including countries of export)
- Recall Strategy
- Indicate the level in your distribution chain you will extend the recall to, if it’s not to the end-user or retail level, indicate why
- Indicate scope of recall and how your strategy can expand if the scope of the recall expands
- Describe your recall effectiveness check strategy and how you will track and communicate with those who are non-responsive
- Method of recall communication
- Written communication is ideal, so companies have records
- Consider posting the recall on your website or social media, if appropriate8
Evaluating and Terminating the Recall
Recall Effectiveness Checks
A recall effectiveness check indicates how well recall communication was received and followed. In the event of an ineffective recall, appropriate steps should be followed in order to increase its effectiveness. This may involve using alternative means of contacting your customers or sending out a follow-up communication that better identifies the product and reason for recall, providing better instructions to customers. This calculation will be important to update as your recall progresses.
Recall effectiveness can be calculated based on the following equation:
(# of cases recovered / # of cases produced) x 100 = Recall Effectiveness
Recall Status Reports
The current state of the recall should be surveyed and updated on a monthly basis, or as directed by regulatory partners, until the recall is terminated. Work with your Regulatory Recall Team to establish the frequency of Status Reports for your recall event.
Topics to investigate could include:
- Dates and method of customer notifications
- Number of customers notified
- Number of customers that responded
- Quantity of recalled product returned or otherwise accounted for
- Number of customers that did not respond (FDA may ask for the identity of such customers)
- Estimated time frame for completion of the recall
- Details of your recall effectiveness checks
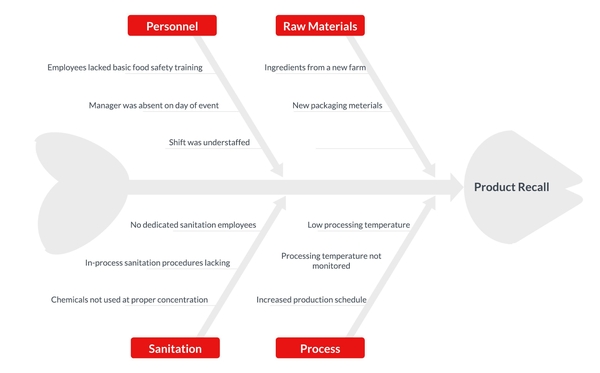
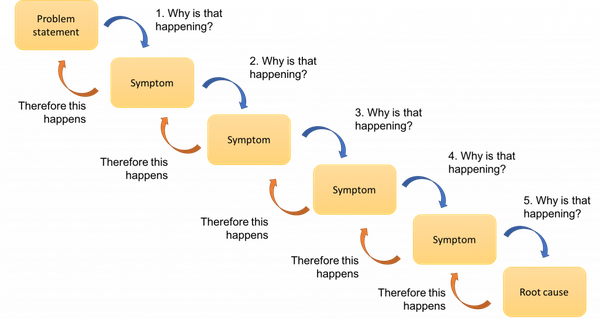
Root Cause Analysis (RCA)
A root cause analysis is used as a retrospective investigational tool to determine the cause or trigger of a food safety event with the goal of finding actions to eliminate the root cause and prevent it from happening again. Visualization tools like Ishikawa or fishbone diagrams, as seen in Figure 4., or the 5 Why's RCA, as seen in Figure 5., can be helpful as a brainstorming tool as your recall and food safety teams try to find and rectify the cause of the recall9.
Corrective Actions and Plans for the Future
All corrections and corrective actions should be documented in real time. This includes a log of all decisions made throughout the recall. Companies must be able to provide written documentation for future inspections and audits.
The Food Safety Plan for the operation must be reanalyzed after a recall event. This may result in changes or updates to the Plan, retraining of employees to increase implementation effectiveness or other actions to prevent reoccurrence of the problem.
Corrective Action Documentation Considerations
Date of record
Code codes effected
Date & time of deviation
Description of deviation
Actions taken to restore the process
Signature of responsible individual
Amount of product involved in the deviation
How is the company evaluating the deviation
Disposition of product
Termination of the Recall
Termination of the recall can occur when it's reasonable to assume the product subject to recall has been removed from commerce. This assessment must be aligned with the degree of hazard in the recalled product and should be a decision made by the recalling company only at the completion of all follow-up and analysis by the Recall Team is completed.
Glossary
Consignee
Consignee means anyone who received, purchased, or used the product being recalled.
Correction
Correction means repair, modification, adjustment, relabeling, destruction, or inspection (including patient monitoring) of a product without its physical removal to some other location.
Direct Account
Direct Account, for the purpose of this guidance, means the first consignee in a firm’s distribution chain.
Initiation of a Recall
Initiation of a recall, for the purpose of this guidance, means a recalling firm’s first communication about a recall, to its direct accounts or to the public.
Market Withdrawal
Market withdrawal means a firm’s removal or correction of a distributed product which involves a minor violation that would not be subject to legal action by the FDA or which involves no violation, e.g., normal stock rotation practices, routine equipment adjustments and repairs, etc.
Recall
Recall means a firm’s removal or correction of a marketed product that the FDA considers to be in violation of the laws it administers and against which the Agency would initiate legal action, e.g., seizure. Recall does not include a market withdrawal or a stock recovery.
Recall classification
The numerical designation (i.e., I, II, or III) assigned by FDA to a particular product recall to indicate the relative degree of health hazard presented by the product being recalled.
| Class I | A situation in which there is a reasonable probability that the use of, or exposure to, a violative product will cause serious adverse health consequences or death. |
|---|---|
| Class II | A situation in which use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences, or the probability of serious health consequences is remote. |
| Class III | A situation in which use of, or exposure to, a violative product is not likely to cause illness or injury. |
| Market Withdraw | When a product has a minor violation that would not be subject to regulatory action |
| Stock Recovery | A company’s removal or correction of a product that has not been marketed or that has not left the direct control of the company. |
Recalling Firm
Recalling firm means the firm that initiates a recall or, in the case of an FDA-requested recall, the firm that has primary responsibility for the manufacture and marketing of the product to be recalled.
Resources
FDA Industry Guidance For Recalls
- Guidance documents
- Regulatory documents
- Model press releases
- Recall coordinators
- Effectiveness check documents
FDA Recalls, Market Withdrawals, & Safety Alerts
- Press releases and public documentation for recalls in the United States
- FDA Recall email listserv
References
- FDA Recall Dashboard. (2023). ↲
- Food and Drug Administration. (2018). Guidance for industry and FDA staff: Questions and answers regarding mandatory food recalls. U.S. Food And Drug Administration. ↲
- FDA Office of Regulatory Affairs. (2018). FDA orders mandatory recall for kratom products due to risk of salmonella. U.S. Food And Drug Administration. ↲
- FDA Office of Regulatory Affairs. (2022). Initiation of voluntary recalls under 21 CFR part 7, subpart c. U.S. Food And Drug Administration. ↲
- FDA Office of Regulatory Affairs. (2014). Listeria monocytogenes Model Press Release. U.S. Food And Drug Administration. ↲
- FDA Office of Regulatory Affairs. (2020). ORA Recall Coordinators. U.S. Food And Drug Administration. ↲
- FDA Office of Regulatory Affairs. (2014). Salmonella Model Press Release (all serotypes). U.S. Food And Drug Administration. ↲
- FDA Office of Regulatory Affairs. (2020). Product recalls, including removals and corrections. U.S. Food And Drug Administration. ↲
- Schrick, S. (n.d.). A guide to conducting root cause analysis in food manufacturing. ↲
Publication date: March 19, 2024
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.