Summary
Grapevine red blotch disease (GRBD) and grapevine leafroll disease (GLRD) are the two economically most significant grapevine virus diseases worldwide. Both diseases can be transmitted through planting material and insects, leading to reduced berry and wine quality and affecting yield and vine growth. To evaluate the distribution of grapevine viruses in North Carolina, a survey was conducted from 2018 to 2020 in vineyards of the Yadkin Valley American Viticultural Area (AVA), the Crest of the Blue Ridge Henderson County AVA, and several other counties in North Carolina. Samples from 280 individual vines were collected and analyzed for the presence of grapevine leafroll-associated viruses (GLRaV) and grapevine red blotch virus (GRBV). Grapevine red blotch virus, the causal agent of GRBD, was found in all sample areas and in 34.64% of vines. Grapevine leafroll-associated virus was found in only 12.5% of all samples, indicating that GRBV is more widely distributed in North Carolina than GLRaV. Moreover, a fee-based grapevine virus testing services was established within NC State Extension's Plant Disease and Insect Clinic (PDIC) in collaboration with NC State's Micropropagation and Repository Unit (MPRU). In this publication, we describe the results of our survey, the proper identification of GRBD and GLRD symptoms in the field, how to sample for grapevine virus detection, and how to submit samples to the PDIC. We further discuss the best grapevine virus management practices for new vineyards and established mature vineyards and the importance of certified plant material.
Introduction
Viruses are small particles made of deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) and sometimes covered with a protein hull. A virus usually cannot replicate without a host organism. Viruses have the capacity to directly infiltrate a cell in a larger organism and replicate themselves by reprogramming and using cell mechanisms. Like animal and human viruses, plant viruses can cause severe disease in a plant. Grapevines can harbor more than 63 known viruses and viruslike agents (Golino et al. 2013), though not all of those cause disease. However, the plant viruses that cause disease symptoms and that are transmitted efficiently (for example, through insects or transplants) can lead to large, hard-to-control outbreaks and often spread fast through the plant supply chain into vineyards worldwide. Such migration can cause severe economic damages and require costly management. Economically, the most important grapevine virus diseases in the U.S. are GRBD and GLRD. Grapevine red blotch disease is caused by GRBV, a DNA virus belonging to the genus Grablovirus in the family Geminiviridae. The symptoms attributed to GRBD were first described on ‘Cabernet Sauvignon’ at the University of California Oakville Research Station in 2008. Since then, GRBD has been affecting grape production in several states in the U.S. (Krenz et al. 2014), and GRBV was consistently isolated from symptomatic grapevines (Al Rwahnih et al. 2013) and in germplasm repositories (Al Rwahnih et al. 2015).
Grapevine leafroll disease is associated with infections by GLRaV, a group of viruses belonging to three genera of the family Closteroviridae. Grapevine leafroll-associated virus 3 (GLRaV-3) belongs to the genus Ampelovirus and is considered the economically most important virus in the GLRD complex (Maree et al. 2013). Grapevine leafroll-associated virus 3 can be transmitted through grafting, soft scale insects (Hemiptera: Coccoidea), and mealybugs (Hemiptera: Pseudococcidae).
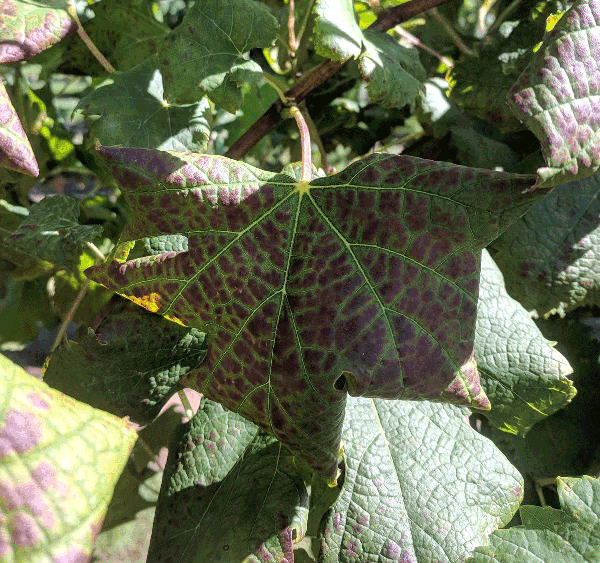
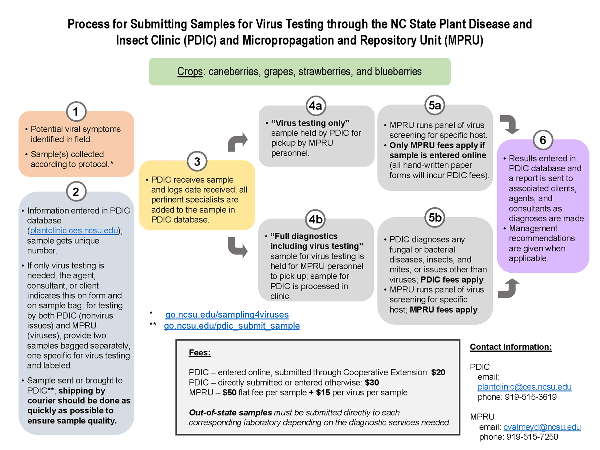
Red cultivars such as Cabernet Franc, Merlot, and Malbec usually display more obvious symptoms of these diseases later in the season, while disease symptoms in white cultivars can often resemble nutrient deficiency or other disorders (Figure 1 and Figure 2) (Cieniewicz et al. 2017). The best time to detect disease symptoms of both GRBD and GLRD is toward the middle to end of the season due to increasing virus titers in the plant tissue. Red cultivars with GRBD symptoms show red leaf veins and no leaf curling (Figure 1), while GLRD symptoms are often associated with red coloration of the leaf tissue but with green leaf veins and curled edges (Table 1; Figure 2). Disease symptoms, however, may vary from vine to vine, and other factors (including trunk disease, cold damage, crown gall, or Pierce's disease) could resemble disease symptoms (Figure 3). Grapevine virus testing is very costly and time intensive. Only after the previously mentioned diseases and disorders are excluded as a diagnosis should a vine be tested for grapevine virus. The PDIC has the capacity to handle grape samples for virus testing through the MPRU. Sample procedures and costs are explained in Figure 8, which is also available to download: Process for Submitting Samples for Virus Testing through the NC State Plant Disease and Insect Clinic (PDIC) and Micropropagation and Repository Unit (MPRU).
|
Virus Name |
Abbreviation |
Disease Name |
Foliar Symptoms |
|
Grapevine Red Blotch Virus |
GRBV |
Grapevine Red Blotch Disease (GRBD) |
In red cultivars, leaves show red veins or have fewer or no rolling edges. Leaf interveins can have red blotches (Figure 1). White cultivars often show chlorosis-like symptoms. |
|
Grapevine Leafroll-associated Virus |
GLRaV-1 to GLRaV-11 |
Grapevine Leafroll Disease (GLRD) |
In red cultivars, there is typically intervein reddening of leaf tissue; veins often remain green; may have rolling edges. Leaves can become full red (Figure 2). White cultivars often show chlorosis-like symptoms (Figure 2). |
Methods
Initial observations over the second half of 2017 in North Carolina vineyards revealed that red grape cultivars exhibited leaf symptoms similar to those associated with GLRD or GRBD (Table 1; Figure 1 and Figure 2). Symptoms included curled leaf edges and reddening of leaves and were observed across several wine-growing regions in commonly grown cultivars—Merlot, Cabernet Franc, Malbec, and Cabernet Sauvignon. From 2018 to 2020, we conducted a vineyard survey in grape-growing regions in North Carolina to assess the incidence and severity of grapevine virus infections. At the same time, we developed a grapevine virus testing service for North Carolina growers. Twenty-eight blocks in fifteen vineyards in three different regions in North Carolina were surveyed for the presence of grapevine viruses in October 2018, October 2019, and November 2020. The vineyards were located in the Upper Hiwassee Highlands American Viticulture Area (AVA), the Crest of the Blue Ridge Henderson County AVA, and the Yadkin Valley AVA. Red cultivars in vineyards were scouted for disease symptoms of GLRD and GRBD (Table 1). Ten to fifteen rows were selected for sampling per vineyard block, and ten symptomatic vines within that block were sampled. Six petioles (from two shoots) were sampled under sterile conditions from each vine, in accordance with the UC Davis Foundation Plant Services (FPS) guidelines. Petioles were sampled with gloves sterilized with 75% ethanol between each sampling. Petioles were put separately in sterile bags after sampling, kept on ice, and sent to the MPRU overnight. RNA for virus detection was extracted the next day. The presence of several grapevine viruses was assessed using molecular methods (RT-qPCR) and confirmed by sequencing either the whole genome (GRBV) or parts of the genome (GLRaV). Samples were tested for the following viruses: GLRaV-2, GLRaV-3, GLRaV-4, GLRaV-7, GRBV, grapevine virus A (GVA), and grapevine virus B (GVB).
Grapevine Virus Disease Symptoms and Distribution in North Carolina
Grapevine red blotch virus was first identified in North Carolina in 2019 (Hoffmann et al. 2019). Grapevine leafroll-associated viruses were first reported in North Carolina in 2020 (Hoffmann et al. 2020). Of all our assessed samples, 34.64 % tested positive for GRBV, 7.86 % tested positive for GLRaV-3, and 4.64 % tested positive for GLRaV-2 (Table 2). Our survey indicates that GRBV is most widely spread in North Carolina, followed by variants of GLRaV.
|
GLRaV-2 |
GLRaV-3 |
GLRaV-4 |
GLRaV-7 |
GRBV |
GVA |
GVB |
|
4.64% |
7.86% |
0% |
0% |
34.64% |
0% |
0% |
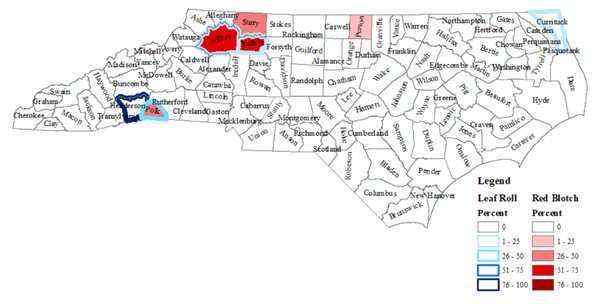
Figure 4. Overview of NC counties in which GRBV and GLRaV were identified. Blue lines indicate the presence of GLRaV (Currituck, Henderson, Polk, Wilkes, and Yadkin), and red shading indicates the presence of GRBV (Person, Polk, Surry, Wilkes, and Yadkin)
N.C. Department of Environmental Quality online GIS of NC Counties for 2019
Potential Vector Mechanisms
Viruses can be introduced into a vineyard though infected plant material. Grapevine red blotch virus and GLRaVs can be transmitted through grafting or propagation. To reduce the risk of infection, practices such as early ordering, developing relationships with nurseries, and selecting a nursery that is part of a certification program are very important. Those practices are described in more detail later.
GRBV and GLRaVs also can be transmitted via insect vectors. These are often the mechanisms that spread the virus within a vineyard from infected plant material to uninfected plant material. Some insect vectors can also be spread through tools, boots, trucks, tractors, and machinery such as hedgers or leaf removers.
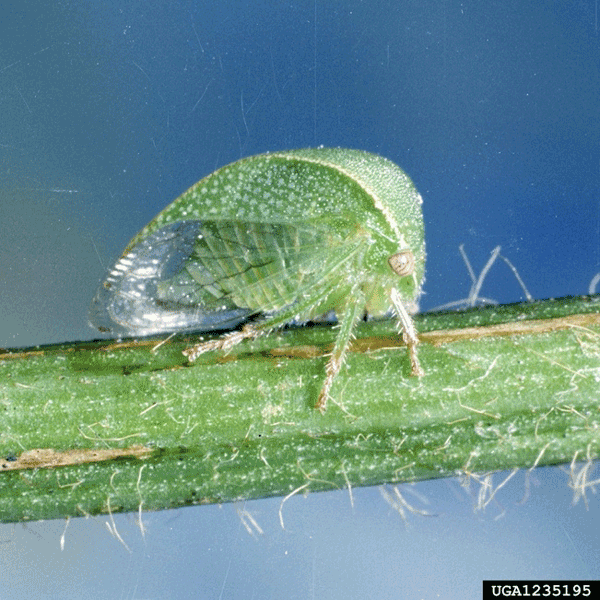
While GRBD and GLRD are both caused by viruses, the two viruses are vectored by different groups of insects. Grapevine red blotch virus has been detected in several species of hemipteran insects (Cieniewicz et al. 2018), but transmission of GRBV in grapes has so far only been documented with the threecornered alfalfa hopper, Spissistilus festinus (Say) (Hemiptera: Membracidae) (Bahder et al. 2016; Flasco et al. 2021).
Threecornered alfalfa hoppers are native to the southern United States. The adults are mostly green, wedged-shaped insects with membranous wings that are about ¼ inch long. The nymphs are also green and wedge-shaped but are wingless and have projections (spines) along the top of their body. Both adult and nymph threecornered alfalfa hoppers (Figure 5) have piercing-sucking mouthparts that they use to feed on the sap of hosts like alfalfa, soybean, cowpea, clovers, and grape. Characteristic feeding by the hoppers often girdles plant stems and petioles through a series of punctures circling the stem or petiole, which can disrupt the movement of nutrients and water within the plant. More important, the piercing-sucking mouthparts allow for the transmission of GRBV directly into the grape plants. As such, the threecornered alfalfa hopper can be considered a pest of major concern in grape production.
Similar to GRVB, GLRaV has been detected in several species of hemipteran insects (Tsai et al. 2010; Maree et al. 2013), particularly mealybugs (Pseudococcidae) and soft scales (Coccidae) (Herrbach et al. 2016). The invasive vine mealybug, Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae), is a key vector of GLRaV but is not currently found in North Carolina. However, the native grape mealybug, Pseudococcus maritimus (Ehrhorn), is common in the Southeast and can also transmit GLRaV (O’Hearn and Walsh 2021).
Grape mealybug adults (Figure 6) are flat, oval-shaped, and about 1/5 inch long with a white, waxy covering and filaments circling the body. Longer filaments from the posterior end make these mealybugs appear to have "tails." In spring, the immature grape mealybugs move toward the base of spurs or under the loose bark of canes, then onto expanding green shoots. During summer, they move out to the green portions of the vine to feed on fruit and foliage. As a byproduct of their feeding, grape mealybugs produce a sweet, sticky liquid called honeydew. The sugary honeydew can become a medium for the growth of sooty mold, a dark, threadlike fungus that creates a black mat on fruit, foliage, and other plant surfaces. Note that the honeydew may also attract ants, which are known to “farm” and protect mealybugs for access to this sugar source. Ants on vines may be a sign of mealybug infestation. Because of its contribution to sooty mold growth and, more important, transmission of GLRaV, grape mealybug is a key pest in southeastern grape production.
Because mealybugs have been relatively rare in North Carolina, we have assumed that much of the GLRD we discover originated at the plant source. To correctly identify species, growers should contact the horticulture agent at their local Cooperative Extension center to assist with proper collection of samples. Sending samples to the PDIC is the fastest means of processing them. Physical samples are preferrable to photographs, as mealybugs must be observed under a microscope to determine the correct species. Samples should consist of infested plant tissue (leaves or fruit) with mealybug adults present. These should be stored in sealed containers and shipped to the PDIC. Ice packs can be included to keep insects from overheating.
When to Sample for Viruses
Disease symptoms related to a grapevine virus infection are usually nonspecific and can easily be confused with symptoms of other physical or biological factors. Disease symptoms of both GRBD and GLRD are more severe in the latter half of the season, and sampling should always occur after véraison. Follow these steps before requesting virus diagnostics:
- Do you see any leaf reddening (red cultivars) or chlorosis (white cultivars)? (See Figures 1 and 2.) If so, you should continue to the next step. If you don’t see any reddening or chlorosis, it is very unlikely that your vine is affected by GRBD or GLRD.
- Inspect the vine for any physical damage to trunk or cordon. If severe damage is observed, the symptoms very likely are related to those structural damages, even if damages are several years old.
- If no structural damage is observed, submit petiole samples from the affected vines to the North Carolina Department of Agriculture & Consumer Services (NCDA&CS) Agronomic Services to acquire a tissue nutrient analysis. Nutrient deficiencies can resemble virus infections.
- After excluding physical and nutritional problems, inspect your vines for the presence of crown gall. Crown gall is usually visible at the trunk base and also sometimes in the cordon area. If a severe crown gall infection is diagnosed, it is very likely that the reddening/chlorosis is related to this infection.
- If crown gall can be excluded, inspect for the presence of grapevine trunk diseases. For this step, cutting into the wood of the cordon or a spur is necessary. Grapevine trunk diseases are fungal pathogens but can cause disease symptoms similar to grapevine viruses. Please talk to your Extension agent before making a diagnosis.
- After ruling out grapevine trunk diseases, acquire whole leaf samples, send them to the PDIC to test for the presence of Xylella fastidiosa, the causal agent of Pierce's disease, a deadly bacterial grapevine disease.
- If you have ruled out all of the previous factors, take samples for grapevine virus detection. Those samples must not be cross-contaminated, so the sampling procedure is relatively complicated and time intensive. You will have to collect a whole shoot with attached leaves, place each shoot into a separate bag, and ship it in a cooled container to the PDIC overnight. Only the base of the branch should be touched. Details for the sampling procedure follow.
How to Sample for Viruses
The PDIC in collaboration with the MPRU provides testing services for the grapevine viruses discussed here. All samples should be shipped overnight on the day of sampling. We recommend sampling in the morning and shipping samples by noon at the latest from Monday to Wednesday. We don’t recommend shipping samples on a Friday. Follow these instructions for sampling.
- Identify a plant of concern according to the steps described in section 6. Mark this vine with flagging tape or another identifier so that you can locate it if results come back positive or if a new sample is required.
- Do not sample on a Friday. Samples need to be shipped and processed within 24 and 36 hours. Best days to sample are Monday, Tuesday, or Wednesday.
- The PDIC website provides general instructions on how to submit samples. Log in directly to the database to enter the information or print and fill out a paper submission form to include with the sample. Enter the requested information, explaining in the comments section that the sample is being submitted for virus testing. If it is not noted as such, the sample will not be forwarded to the MPRU for virus testing.
- Select a shoot from your chosen vine that displays the most severe symptoms and has living leaf tissue. An example shoot is displayed in Figure 7. Sample only one shoot per vine.
- Remove the entire symptomatic shoot and put it in an individual bag to avoid cross-contamination. It does not matter which part of the plant the shoot is collected from (primary, secondary, or lateral). Touch only the base of the shoot.
- If sampling several vines, place each shoot in a separate, unused plastic bag. The bag should be large enough to cover the whole shoot.
- Repeat steps D through F for as many vines as you wish to test. If sending in more than one sample, please label the bags (for example, 1, 2, 3). Make sure to match the appropriate label to the corresponding PDIC sample form. Flag sampled vines to keep track of your sample numbers and testing results.
- Cool the bags in a refrigerator or a cooled container and ship the samples overnight on the same day of sampling.
- Include the completed PDIC forms with the samples. Samples and forms need to be placed into a shipping container with a cool pack and shipped overnight on the same day of sampling.
- Shipping instructions and addresses are listed on the PDIC website. Please be familiar with the shipping and handling process before sampling your vines.
The diagnosis of grapevine viruses is a molecular method, which requires a significant amount of labor and materials. Therefore, virus diagnosis requires fees for processing samples (Figure 8; Table 3). A submission fee to the PDIC for diagnoses of nonviral issues will cost $20 if entered online and submitted through N.C. Cooperative Extension, and $30 if sent directly to the clinic. For virus testing, each sample will cost $50 for molecular laboratory processing, in addition to other diagnostic fees previously mentioned. All laboratory processing and the testing for viruses will be done at the MPRU. For each virus that is tested for, an additional $15 will be charged. Testing will begin for the most widespread viruses (GRBV and GLRaV-3); if those are negative, testing will continue for other viruses. Diagnosing only viruses requires a minimum of $65 in fees (molecular processing and one virus test). This fee will be billed through the MPRU. All other diagnostics through the PDIC will require additional fees, as will additional viruses tested for at the MPRU.
|
Process |
Tasks |
Fees |
|
Handling and Processing – PDIC |
Sample receiving, processing, distributing. |
Free for virus-only testing. For additional diagnostics (fungal pathogens, insects/mites): $20 – when submitted online and through Extension offices $30 – when submitted directly. |
|
Processing – MPRU |
RNA extraction and storing at -112°F. |
$50 |
|
Testing for Viruses |
Each virus will be charged separately. |
$15 per virus |
Grapevine Virus Management
Currently there is no cure for virus-infected grapevines. Any virus-infected vine can potentially be a source for vectors to spread in a vineyard, given the presence of capable vectors and environmental conditions. Since it is not clear how viruses are spread in North Carolina, grapevine virus management in a vineyard is mostly preventative and starts with very careful planning. The first step in prevention is to avoid introducing infected plant material into a vineyard. Many nurseries are part of a voluntary virus-free certification processes. For California-based nurseries, this process is the California Department of Food and Agriculture (CDFA) Grapevine Registration and Certification Program. CDFA certification guarantees that individual vines from increase blocks and nursery blocks will be frequently tested for grapevine viruses. Several nurseries on the East Coast perform similar virus-testing procedures. Ask your nursery when the mother wood was last tested for viruses. Some nurseries will let you visit to sample plant material for independent testing from the increase block that produces your plants, either before or after placing your order. For this type of inspection, however, you must place your order at least 15 to 18 months before your planned planting date to allow time for propagation.
Nursery certification processes, early ordering, and a potential visit with the nursery decrease the chances of receiving infected plant material as a grower, but require early planning. Even a certification process is not a guarantee that each vine received will be virus-free. It is therefore important to test your plant material BEFORE planting. We recommend taking random wood samples of rootstock and scion separately and sending them to the PDIC for virus testing. Please contact your local Extension center ahead of time to schedule a site visit and a time slot for sampling. Inspect the planting material for the presence of mealybugs and other insect vectors carefully before planting. It is also important to know the history of your site. If you plant on a site that was infected with GLRaV before, you must remove as many roots as possible.
Newly planted vineyards need to be scouted frequently for virus symptoms. Treat any newly planted vineyard as if it is virus-infected and include the necessary spray applications in the first three to four years to control vectors (see the 2021 Southeast Regional Bunch Grape Integrated Management Guide for instructions). Remove every vine that is confirmed to be infected with a virus immediately. Grapevine red blotch virus can often be latent in rootstocks, and disease symptoms can become apparent after a few years. If you have a mature vineyard in North Carolina with grapevine virus-infected vines, replacing vines can be difficult. However, if virus-infected vines remain in the vineyard, the potential to infect other vines in your and neighboring vineyards becomes higher. We recommend removing infected vines and filling gaps in the vineyard either with replants or by extending the cordon using neighboring vines. At the same time, it is important to spray as necessary to control insect vectors.
Conclusions
Grapevine red blotch virus and GLRaV are widespread in Vitis vinifera vineyards of North Carolina. Both viruses can cause early vine decline, decrease fruit and wine quality, and lower yields, therefore leading to considerable losses. The PDIC and the MPRU have established a virus testing service for growers in North Carolina. This fee-based testing service can be used to diagnose plant material for the presence of grapevine viruses. In mature vineyards, virus symptoms of the associated diseases can be easily confused with other disorders and need to be identified properly. For vineyard establishment, the choosing of certified planting material, the development of good relationships with the nursery, and the responsible testing of material before planting are highly recommended.
Acknowledgments
This study was partly funded by the N.C. Wine and Grape Council. We thank all of the vineyard owners and vineyard managers who have participated in this study and allowed us to take samples from their vines. We further thank Mysore Sudarshana, USDA-ARS in Davis, CA, for his support and help establishing molecular protocols at the MPRU; Stephanie Bolton (Lodi Winegrape Commission) for the helpful support and supply of educational material; N.C. Cooperative Extension agents Elina Snyder, Hannah Lepsch, and Karen Blaedow for their support and help collecting samples in the vineyards; and Tekan Rana, University of Maryland, for collection of samples in the first year of this study.
Citations
Al Rwahnih, M., A. Dave, M.M. Anderson, A. Rowhani, J.K. Uyemoto, and M.R. Sudarshana. 2013. "Association of a DNA Virus with Grapevines Affected By Red Blotch Disease in California." Phythopathology 103, no. 10: 1069–76.
Al Rwahnih, M., A. Rowhani, and D. Golino. 2015. "First Report of Grapevine Red Blotch-Associated Virus in Archival Grapevine Material from Sonoma County, California." Plant Disease.
Bahder, B.W., F.G. Zalom, M. Jayanth, and M.R. Sudarshana. 2016. "Phylogeny of Geminivirus Coat Protein Sequences and Digital PCR Aid in Identifying Spissistilus festinus as a Vector of Grapevine Red Blotch-Associated Virus." Phytopathology 106, no. 10: 1223–1230.
Cieniewicz, E.J., S.J. Pethybridge, G. Loeb, K. Perry, and M. Fuchs. 2018. "Insights into the Ecology of Grapevine Red Blotch Virus in a Diseased Vineyard." Phytopathology 108, no. 1: 94–102.
Cieniewicz, E., K. Perry, and M. Fuchs. "Grapevine Red Blotch: Molecular Biology of the Virus and Management of the Disease." In Grapevine Viruses: Molecular Biology, Diagnostics and Management, by Meng, Martelli, Golino, and Fuchs, 303–314. Switzerland: Springer, 2017.
Golino, D., A. Rowhani, and J.K. Uyemoto. "Grapevine Virus Diseases." In Grape Pest Management, Publication 3343, edited by Larry J. Bettiga, 157–173. Oakland, CA: University of California Agriculture and Natural Resources, 2013.
Flasco, M., V. Hoyle, E. Cieniewicz, B. Roy, H. McLane, K.L. Perry, G.M. Loeb, B. Nault, and M. Cilia. 2021. "Grapevine Red Blotch Virus is Transmitted by the Three-cornered Alfalfa Hopper in a Circulative, Nonpropagative Mode with Unique Attributes." Phytopathology.
Herrbach, E., J. Le Maguet, and G. Hommay. 2016. "Virus Transmission by Mealybugs and Soft Scales (Hemiptera: Coccoidea)." In Vector-mediated Transmission of Plant Pathogens, edited by J. K. Brown, 147–161. St. Paul, MN: American Phytopathological Society Press, 2016.
Hoffmann, M., W. Talton, M. Nita, T. Jones, M. Al Rwahnih, M.R. Sudarshana, and C. Almeyda. 2019. "First Report of Grapevine Red Blotch Virus, the Causal Agent of Grapevine Red Blotch Disease, in Vitis vinifera in North Carolina." Plant Disease.
Hoffmann, M., W. Talton, M. Nita, T. Jones, M. Al Rwahnih, M.R. Sudarshana, and C. Almeyda. 2020. "First Report of Grapevine Leafroll-associated Virus 3 (GLRaV-3) in Vitis vinifera in North Carolina." Journal of Plant Patholology 103: 385–386.
Krenz, B., J.R. Thompson, H.L. McLane, M. Fuchs, and K.L. Perry. 2014. "Grapevine Red Blotch-associated Virus is Widespread in the United States." Phytopathology 104, no. 11: 1232–40.
Maree, H.J., R.P.P. Almeida, R. Bester, K.M. Chooi, D. Cohen, V.V. Dolja, M.F. Fuchs, D.A. Golino, A.E.C. Jooste, G.P. Martelli, R.A. Naidu, A. Rowhani, P. Saldarelli, and J.T. Burger. 2013. "Grapevine Leafroll-associated Virus 3." Frontiers in Microbiology 4: 82.
O'Hearn, J.S. and D.B. Walsh. 2021. "Grapevine Leafroll-associated Virus 3 Vectored by Pseudococcus maritimus at Low Population Levels." Journal of Entomological Science 56, no. 1: 106–110.
Tsai, C.W., A. Rowhani, D.A. Golino, K.M. Daane, and R.P.P. Almeida. 2010. "Mealybug Transmission of Grapevine Leafroll Viruses: An Analysis of Virus-vector Specificity." Phytopathology 100, no. 8: 830–834.
Publication date: Dec. 1, 2021
AG-911
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.