Situation in North Carolina
Nearly all North Carolina soils are naturally acidic and need lime, which neutralizes the acidity, for optimum growth of crops, forages, turf, trees, and many ornamentals. Even though most of these soils have been limed in the past, periodic additions of lime based on soil tests are still needed. Field records compiled by the Agronomic Division of the NC Department of Agriculture & Consumer Services (NCDA&CS) emphasize that poor management of soil pH (the number that indicates whether soil is acid or alkaline) accounts for a high percentage of the “crop problems” they diagnose. Also, soil-test summaries compiled by NCDA&CS verify the need for lime. During the fiscal years 2019 and 2020, 431,000 soil test reports from mineral soils cultivated with cotton, grains, and peanut were analyzed at the NCDA&CS soil testing lab. A review of these results found that 37 percent of samples presented pH levels below 5.8, and these fields would benefit substantially from liming. In addition, about 9 percent of these samples presented a pH greater than 6.5, indicating that those fields had been overlimed.
Although response to lime is frequently subtle (in contrast to the quick greenup that a nitrogen application gives to corn), ignoring its regular use limits crop yields. Proper use of lime, in combination with other sound agronomic and pest control practices, should increase crop income in North Carolina. Using conservative estimates based on 2021 North Carolina Agricultural Statistics, a 5 percent yield increase from proper lime use on crops that are sensitive to low pH (cotton, soybeans, peanuts) would increase gross farm income by about $67 million. In addition, a 1 percent yield increase from proper lime use on crops less sensitive to low pH (tobacco, corn, commercial vegetables, wheat, fruits/nuts) would increase gross farm income by about $16 million.
Nature and Cause of Soil Acidity
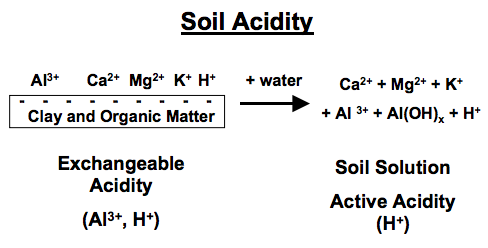
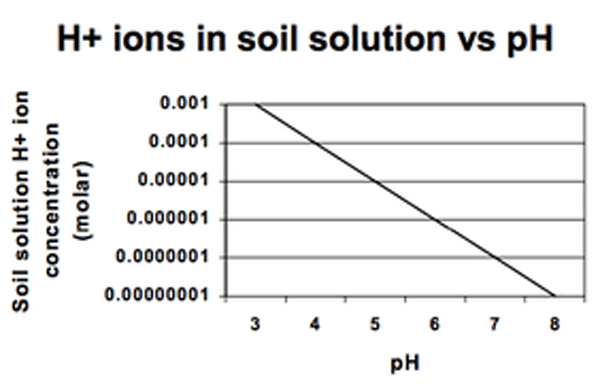
“Soil acidity” is the term used to express the quantity of hydrogen (H+) and aluminum (Al3+) cations (positively charged ions) in soils (Figure 1), and soil pH is an indicator of acidity. The pH is the negative logarithm of the hydrogen concentration, expressed on a scale from 1 to 14 (Figure 2a and Figure 2b). A pH of 7.0 is defined as neutral, with values below 7.0 being acidic and above 7.0 being basic or alkaline. Because the pH scale is logarithmic, soil with a pH of 5 is 10 times more acidic than soil with a pH of 6 and is 100 times more acidic than soil with a pH of 7. Greater amounts of lime are needed to raise soil pH to a given level at lower pH values. Root growth and plant development may be severely restricted if acidic cations, especially aluminum, occupy a large percentage of the negatively charged cation exchange capacity (CEC). This negative charge is due to the chemical makeup of the soil clay and organic matter and means that it can attract positively charged ions.
The exchangeable aluminum is in equilibrium with dissolved aluminum in the soil solution and reacts with water to form hydrogen ions in the soil solution:
Al3+ + H2O < — > Al(OH)2+ + H+
The larger the percentage of exchange sites occupied by aluminum, the greater the amount of hydrogen formed, thus the lower the pH and the higher the acidity of the soil. Over time, soils become more acid due to the leaching of calcium (Ca2+) and magnesium (Mg2+), which is especially pronounced in sandy coastal plain soils. Acidification also occurs when hydrogen is added to soils by decomposition of plant residues and organic matter and during the nitrification of ammonium added to soils as fertilizer (UAN solutions, urea, ammonium nitrate, ammonium sulfate, anhydrous ammonia), manures, or plant residues:
NH4+ + 1 1⁄2 O2 ➜ NO3- + 4 H+
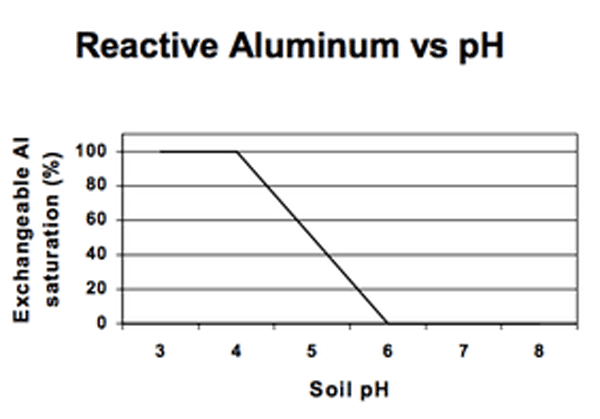
The H+ added to soils reacts with the clay minerals (aluminum silicates) and releases Al3+, which goes on to the exchange sites, contributing to soil solution acidity, as noted above. Soil pH also influences the concentration of many dissolved ions in the soil solution, including aluminum, which decreases in concentration as soil pH increases (Figure 2c).
Benefits of Proper Lime Use
Proper liming provides a number of benefits:
- Nutrient solubility and availability are improved by higher soil pH. The optimal pH range for most nutrients in North Carolina mineral soils is between 5.8 and 6.2. Manganese is an example of a micronutrient required by plants that becomes less soluble as pH increases. It is available, and can even occur at toxic concentrations, if the pH is too low. It becomes insoluble and unavailable if the pH is too high, and deficiencies can result.
- Plants develop healthier roots because they are exposed to reduced toxicity of aluminum and manganese (most piedmont and mountain soils). Better root growth may improve nutrient uptake and enhance drought tolerance.
- Lime is an economical source of essential calcium (as well as beneficial magnesium if dolomitic limestone [see Liming Materials] is used). Furthermore, these nutrients are released slowly over a period of three to four years and may be better protected from leaching than those supplied by more soluble fertilizers.
- Fertilizer phosphorus (P) is used more efficiently. Aluminum at a pH of less than 5.4 is chemically active and combines with fertilizer phosphorus, causing it to become less soluble. Because this fertilizer phosphorus is tied up, less is available to the next crop. In some instances, fertilizer phosphorus inadvertently serves as a liming material by decreasing aluminum solubility.
- Increased soil CEC occurs, as well as reduced leaching of basic cations, particularly potassium. The soil CEC includes a number of pH-dependent sites that become available to hold cations as the pH increases. When these sites are occupied by strongly attached aluminum (low pH), any potassium added in fertilizer is more susceptible to leaching. Proper liming will minimize, but not completely prevent, leaching of potassium. Soils with deep sandy surfaces are particularly susceptible.
- Nodulation of legumes is enhanced, which improves nitrogen fixation. The bacteria (Rhizobia) in nodules on legume roots (soybeans, peanuts, alfalfa, and clover) synthesize greater amounts of nitrogen from the soil atmosphere for use by the legume in places where soil pH is not too low. Nitrogen fixation provides an economical source of nitrogen and may supply the succeeding crop with substantial residual nitrogen. In addition, molybdenum (Mo), an element essential to Rhizobia in the nitrogen-fixing process, is increasingly tied up as soil pH of mineral soils gradually declines below 5.5. Therefore, less-than optimum molybdenum levels may result in reduced growth of legumes, such as soybeans, peanuts, and clovers.
- Triazine herbicides, such as atrazine and simazine, work better in a higher pH environment.
- Some nematicides also may work better.
- Optimal pH allows the breakdown of some herbicides, preventing damage to rotational crops.
Soil Testing and Target pH to Determine Lime Rates
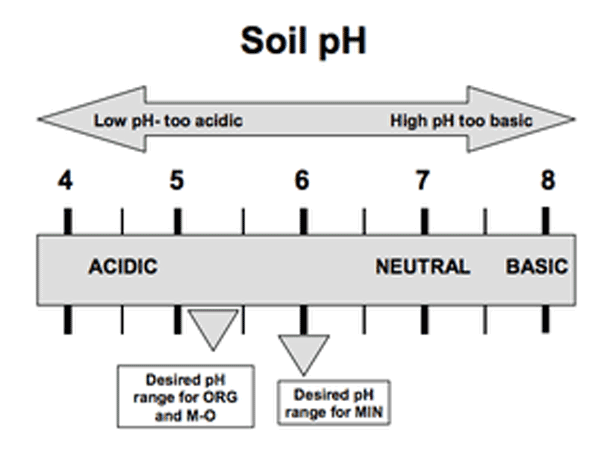
Lime recommendations must take into account differences in acidity among soils as well as differences among various crops’ tolerance to acidity. It is important to remember that the optimum pH is not the same for all crops or soils. For example, on most Midwestern soils most crops grow best at a pH of 6.5 to 7.0. These values would cause micronutrient deficiencies in parts of North Carolina. Many micronutrients become less soluble as the pH increases, reducing their availability to plants. For example, manganese deficiencies frequently occur following overliming in many North Carolina soils. North Carolinians also must remember the state has three different soil classes—organic (ORG), mineral (MIN), and mineral-organic (M-O).
NCDA&CS makes soil class determination using two criteria: the amount of humic matter (HM) and the soil density (weight/volume ratio, W/V). The state has a substantial amount of acreage in organic soils, primarily in the east. Organic matter complexes or ties up aluminum; consequently, plant growth is possible at lower pH levels in these soils than in mineral soils. In North Carolina, very little aluminum is left in solution at pH 6 in mineral (MIN) soils, at pH 5.5 in mineral-organic (M-O) soils, and at pH 5 in organic (ORG) soils.
Organic matter also has a relatively high CEC, meaning a greater quantity of lime is needed to raise pH than when dealing with mineral soils. Since organic soils contain less of many micronutrient cations, overliming can cause additional micronutrient deficiencies. For these reasons, organic and mineral soils differ in recommended or target pH. For most commonly grown field crops, mineral soils have a target pH of 6.0. For mineral-organic soils, the target pH is 5.5; for organic soils it is 5.0 (Table 1).
| Soil Class | Humic Matter (%) and Weight/Volume Ratio (g/ml) Criteria |
Target pH (most crops) |
|---|---|---|
| Mineral (MIN) | HM ≤ 3.37 and W/V > 0.5 | 6.0 |
| Mineral-Organic (M-O) | HM ≤ 3.37 and W/V ≤ 0.5 or 3.37 < HM ≤ 5.23 and W/V > 0.5 |
5.5 |
| Organic (ORG) | 3.37 < HM ≤ 5.23 and W/V ≤ 0.5 or HM > 5.23 |
5.0 |
Another issue to consider is that different soil laboratories may use different testing methods for their particular soil conditions. The NCDA&CS laboratory reports both the soil pH and the “Ac value.” The “Ac value” is a measure of the exchangeable acidity, which is the combined total of exchangeable aluminum and reactive hydrogen ions (Figure 1). Both the soil pH and the Ac value are needed to calculate lime applications. Although portable soil test kits determine pH rapidly, it is not possible to make an accurate lime recommendation based solely on a pH measurement. Producers submitting soil samples to other soil test laboratories should ask questions about laboratory methods and target pH assumptions used in determining lime recommendations.
Crops differ in their ability to tolerate a low pH, with optimum values ranging from 4.5 to 6.5 (Table 2). Plants such as blueberries, azaleas, and native ornamentals are especially tolerant of, and grow better at, low pH. In contrast, plants such as alfalfa, cotton, and tomatoes grow better at a higher pH.
| Plant group | Target pH | Species | |
|---|---|---|---|
| Field crops | 6.0 | Corn, millet, small grains, sorghum, soybeans, tobacco | |
| 6.2 | Cotton | ||
| Vegetables | 6.0 | Beans, cucurbits, cole crops, potato, spinach, sweet potato | |
| 6.5 | Asparagus, tomato | ||
| Small fruits | 4.5 | Blueberry | |
| 6.0 | Blackberry, grape, strawberry | ||
| Forage grasses | 6.0 | Fescue, orchardgrass, timothy (maintenance), bahiagrass, bluegrass, sudangrass | |
| 6.5 | Fescue, orchardgrass, timothy (establishment), bermuda | ||
| Forage legumes | 6.0 | Crimson and white clover, lespedeza | |
| 6.5 | Alfalfa, ladino, red clover | ||
| Lawns/gardens | 5.0 | Azalea, camelia, mountain laurel, rhododendron | |
| 5.5 | Centipedegrass | ||
| 6.0 | Other lawn grasses, flower garden, shrubbery, shade trees | ||
| 6.5 | Rose, vegetable garden | ||
| Nursery | 5.0 | Ginseng, native ornamentals, rhododendron | |
| 6.0 | Most other flowers | ||
| 6.5 | Gypsophila | ||
| Trees/Orchards | 5.5 | Fir/Northern spruce Christmas trees, pine | |
| 6.0 | Apple (maintenance), pecan, hardwoods | ||
| 6.2 | Peach (maintenance) | ||
| 6.5 | Apple/peach (establishment), red cedar/blue spruce Christmas trees | ||
| * Target pH is lower for M-O and ORG soils, 5.5 and 5.0, respectively, for crops that have optimum pH of 6.0–6.5 on MIN soils. | |||
Because of these differences in crops and soils, the NCDA&CS soil-test report uses the following equation to calculate the amount of lime needed to achieve the target pH for the particular soil class and crop combination under consideration:
\(\mathrm{Lime\ }\left(\mathrm{ton/acre}\right)\ =\ \mathrm{\mathrm{Ac}}\ \times\ \ \left[\left(\mathrm{target\ pH}\ -\mathrm{current\ pH}\right)\div\left(6.6-\mathrm{current\ pH}\right)\right]-\mathrm{RC}\)
The Ac value and target pH have already been discussed. The current pH is the pH of the sample analyzed. Since some lime applied within the past 12 months may not have fully reacted, “residual credit” (RC) is given to applied lime, depending on the soil class and how recently it was applied. Residual credit is reduced by 8 percent per month from the time of application to the time of soil sampling for mineral soils and by 16 percent per month for mineral-organic and organic soils.
Example for a Mineral Soil
If current soil pH = 5.0, target pH = 6.0, Ac = 1.2, and RC = 0 (since no lime was applied within the past year), the lime recommendation is:
\(1.2\times\left[\left(6.0-5.0\right)\div\left(6.6-5.0\right)\right]-0=0.76\mathrm{\ ton/acre}\)
This value is rounded off and reported as 0.8 ton/acre.
For a more acid-tolerant crop, such as Fraser fir Christmas trees with a target pH of 5.5, the recommended lime rate would be lower:
\(1.2\times\left[\left(5.5-5.0\right)\div\left(6.6-5.0\right)\right]-0=0.38\textrm{ ton/acre}\), rounded off and reported as 0.4 ton/acre
Soil testing through NCDA&CS offers recommendations for two years, growing either the same crop or different crops. If two different crop recommendations with different target pH are requested, lime rates are calculated for both crops but are given with the highest of the two lime rates suggested for the first crop. No additional lime is recommended for the second crop. Lime rates are reported in tenths of a ton, with no lime recommended when calculations indicate less than 0.3 ton. However, if the calculation indicates that no lime is needed, but the soil pH is 0.3 unit or less below the level desired, an application of 0.3 ton per acre or 15 pounds per thousand square feet is recommended. All lime rates are based on the use of a standard agricultural lime (see Adjusting Lime Rate Based on Effective Neutralizing Value).
If soil pH is too high for the desired crop, elemental sulfur (S) is the most effective soil acidulant. The amount of acidity generated by 640 pounds of elemental S is the same as that neutralized by 1 ton of lime.
S0 + H2O + 3/2 O2 ➜ 2 H+ + SO42- (sulfuric acid produced)
Several weeks may be required for pH changes, depending on temperature and moisture, which control the microbial activity needed for these reactions. The appropriate rate can be approximated, based on the amount of lime required to produce the opposite change in soil pH. For example, if the pH of the soil in the above liming example is to be lowered to a 4.5 pH for blueberry production, reverse the situation and consider the target pH to be 5.0 and the current pH to be 4.5:
\(1.2\times\left[\left(5.0-4.5\right)\div\left(6.6-4.5\right)\right]-0=0.29\mathrm{\ ton\ lime/acre}\)
A reduction in pH can be accomplished by:
\(0.29\times 640\ =186\mathrm{\ lb\ elemental\ S/acre}\)
Soil pH also can be lowered by applying aluminum sulfate or iron sulfate. You should follow the manufacturer’s instructions for appropriate rates. A slight pH reduction can be produced by using ammonium sulfate as a fertilizer source of nitrogen and sulfur.
Liming Materials
Liming materials come in two types. Those containing only calcium carbonate (CaCO3), calcium hydroxide [Ca(OH)2], or calcium oxide (CaO) are called “calcitic limes.” Pure calcium carbonate is used as the standard for liming materials and is assigned a rating of 100 percent. This rating is also known as the “calcium carbonate equivalent,” and it is referred to as the CCE. All other liming materials are rated in relationship to pure calcium carbonate.
The second type of liming material contains significant amounts of magnesium carbonate (MgCO3) in addition to calcium materials and is called “dolomitic lime.” North Carolina law requires that dolomitic lime contain at least 6 percent magnesium in the carbonate form by weight. If a soil is low in magnesium, dolomitic lime should be used; otherwise calcitic lime can be used. Many organic soils and some piedmont soils are naturally high in magnesium, whereas most sandy soils in the coastal plain have little magnesium. The soil-test report will indicate which lime should be used. A magnesium fertilizer could be used instead of dolomitic lime, but the cost of this treatment is almost always considerably higher. Dolomitic limes are slightly more efficient in neutralizing soil acidity and may have CCE values greater than 100, depending on purity.
Liming materials neutralize acidity by dissolving and releasing a base (HCO3- , OH-) into the soil solution, which reacts with acid (H+, Al3+). The chemical reaction of dolomitic lime with soil acidity is as follows:
Equation 1:
Calcium Magnesium Carbonate + Water ➜ Calcium + Magnesium + Bicarbonate + Hydroxide
CaMgCO3 + H2O ➜ Ca++ + Mg++ + 2HCO3- + 2OH-
If dolomitic limestone is used, the calcium or magnesium helps displace the hydrogen and aluminum on the soil exchange sites, and the hydroxyl ions react to neutralize these acidic components as shown in equations 2 and 3. The bicarbonate anion reacts with hydrogen to form a very weak acid.
Equation 2:
Aluminum + Hydroxide ➜ Insoluble Aluminum Hydroxide
Al+3 + 3OH- ➜ Al(OH)3
Equation 3:
Hydrogen + Hydroxide ➜ Water
H+ + OH- ➜ H2O
Aluminum hydroxide is insoluble; therefore the aluminum is temporarily inactivated. Hydrogen is neutralized and water is formed when hydrogen and hydroxide ions combine. In the lime neutralization process, carbon dioxide (CO2) is also given off into the atmosphere.
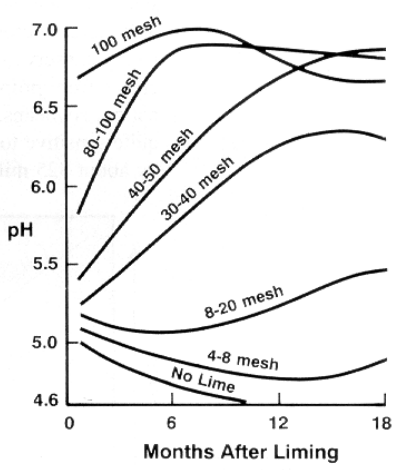
Because lime dissolves very slowly, it must be finely ground to neutralize soil acidity effectively (Figure 3). Lime fineness is measured by using sieves with different mesh sizes. The standard mesh size numbers indicate the number of wires per inch. Thus, higher mesh size numbers signify smaller holes, which limit passage to finer particles. Note that 40- to 50-mesh lime raised the pH to a higher level than 8- to 20-mesh lime did during an 18-month study. Thus, the ability to neutralize soil acidity depends on both the purity (CCE) and the particle size of the liming material. The effective neutralizing value (ENV) is a way to quantitatively evaluate limes based on both purity and particle size. It is calculated by multiplying the CCE (expressed as a decimal) by the relative reactivity (based on fineness). Several private analytical laboratories offer agricultural lime analysis, including both CCE and mesh-size analyses. Consult a Cooperative Extension center for information.
Liming Product Standards
Size and other criteria have been established by the state of North Carolina for the sale of agricultural liming materials to ensure a quality product. They are:
- Agricultural liming materials must be crushed so that at least 90 percent passes through a US standard 20-mesh screen (with a tolerance of plus or minus 5 percent).
- For dolomitic limestone, at least 35 percent of materials must pass through a US standard 100-mesh screen; for calcitic limestone, at least 25 percent must pass through the same screen (both with a tolerance of plus or minus 5 percent).
- A product must contain a minimum of 6 percent magnesium in the carbonate form to be classified as dolomitic limestone.
- There is no minimum CCE requirement for limestone sold in North Carolina. However, the product must be labeled to show the amount necessary to equal that provided by a liming material having a 90 percent CCE. For example, a product having a CCE of 80 percent would be labeled “2,250 pounds of this material equals 1 ton of standard agricultural liming material.”
- Pelleted lime must slake down to the fineness criteria specified above when it comes in contact with moisture.
Lime Form
The most commonly used liming material in North Carolina is finely ground dolomitic rock, but calcitic lime is also widely used. Additional liming materials include burnt lime or hydrated lime, pelleted lime, liquid lime, wood ash, and industrial slags. North Carolina has few good natural lime sources. Calcitic marl liming materials (soft marine shell deposits) are available in the coastal plain, but there are no dolomitic lime deposits in the east. Dolomitic lime is commonly obtained from the mountains of Virginia or Tennessee.
Most agricultural lime is sold in bulk as a damp powder because dry lime is very dusty and difficult to handle and spread. However, lime is occasionally excessively wet.
Lime is sometimes sold in pellet form. The pellets are not large grains of solid limestone, but are formed from lime that has been finely ground. Pellets are less dusty and easier to spread, but they are also more expensive than powdered lime. Pelleted lime comes into contact with fewer soil particles as compared to finely ground lime. As a result, soil pH changes are slower with the pellets. Soil reaction will be enhanced if the ground can be re-tilled several days after the pellets have been mixed into the soil and become soft. Pelleted lime is convenient for landscape use, but it is not economical for most field crops.
Lime is also sometimes sold as a suspension, often called “liquid lime.” It consists of fine lime particles mixed with water and a suspending clay. All the lime particles must be 100 mesh or finer. Up to 1,000 pounds of lime can be suspended in a ton of liquid product. The main advantages of liquid lime are ease of handling and precise application. This material, although a fluid, does not react any faster or differently than dry lime of the same particle size. All of the lime in a suspension is fast acting, and a ton of product (1,000 pounds of fine lime particles plus clay and water) will raise the pH as fast as a ton of dry lime. However, due to particle size and enhanced initial reactivity, the effectiveness of suspensions is short-lived, compared to regular agricultural limestone, and liming will probably have to be repeated every year. There also may be some potential for raising soil pH slightly above the target if these materials are used. In the end, using suspensions to correct soil acidity is considerably more expensive.
Occasionally, industrial byproduct liming materials or materials considered as waste with lime value become available. If the neutralizing value is known and the material is ground finely enough to react in the soil, these can be economical substitutes. Wood ash, steel mill slag, lime- stabilized biosolids, and poultry litter are examples. Often such materials contain other plant nutrients; however, most wood ash products are known to contain little if any magnesium. Magnesium is an important consideration if used on coarse-textured, low CEC soils where leaching occurs. These products must meet the legal standards noted above to be sold as liming materials in North Carolina. Even if they do not meet all of the standards, they still may be useful as soil amendments capable of reducing soil acidity and supplying a variety of nutrients, including calcium, magnesium, potassium, phosphorus, and micronutrients. If a product does not meet all the specifications of the lime law, the supplier will not be able to make claims about liming effectiveness. Then it will be up to the purchaser to have the material tested. Each lot of such materials should be analyzed, as considerable variation in CCE and fineness may occur. As with conventional lime, the ENV needs to be known in order to determine the appropriate application rate. The NCDA&CS Agronomic Division waste laboratory can analyze these materials for CCE. From the CCE determination, an ag lime equivalent (ALE) is reported. The ALE does not take into account particle size or fineness. The ALE is a numerical value expressed as the amount of waste in tons per acre that equals one ton of standard agricultural lime. In addition to ALE, the waste analysis provides nutrient value of the waste.
Adjusting Lime Rate Based on Effective Neutralizing Value
All lime rates recommended by the NCDA&CS laboratory are based on a concept of standard agricultural lime with a CCE of 90 percent (0.9) and a fineness meeting the minimum NC lime law requirements for a dolomitic lime (i.e., 90 percent passes a 20-mesh screen and 35 percent passes a 100-mesh screen), so ENV=0.61 (based on equation 2 below). The actual materials available for application vary widely. Calculating the effective neutralizing value (ENV) of a liming material accounts for the two contributing effects (purity and fineness) that determine expected soil pH increase after application. The best equation (Equation 1 below) considers some contribution by material of the 8 mesh size range and finer. (For all calculations, use decimal fractions rather than percentage values.)
Screen the liming material with mesh sizes 8 and 60; this may need to be performed by a private laboratory:
A = proportion of particles between 8 and 60 mesh size (assume 50 percent effective)
B = proportion of particles finer than 60 mesh size (assume 100 percent effective)
Equation 1
\(\mathrm{ENV}=\mathrm{CCE}\times\left[\left(A\times0.5\right)+\left(B\times1\right)\right]\)
Example: A liming material with a CCE of 80 percent (0.80) was found to have 95 percent of particles finer than 8-mesh, 90 percent finer than 20-mesh, 50 percent finer than 60-mesh, and 25 percent finer than 100-mesh.
Using equation #1:
A = 45% (0.45) because 95% (finer than 8-mesh) minus 50% (finer than 60-mesh) equals 45% (between the 8- and 60-mesh sizes).
B = 50% (0.5)
\(\mathrm{ENV}=0.80\times \left[\left(0.45\times0.5\right)+\left(0.5\times1\right)\right]=0.80\times 0.725=0.58\)
The actual application rate of a liming material can then be calculated from the soil-test recommendation, assuming that standard agricultural lime has an effective neutralizing value of 61 percent (0.61). Compared to standard agricultural lime, \(0.61\div0.58=1.05.\). Thus 1.05 tons of this material should be used for every 1 ton recommended on the soil test. If this material had been evaluated using just CCE, the lime equivalence would have been calculated as: \(0.9\ \div0.8=1.12\;tons\;product\; per\; ton\; standard\; agricultural\; lime.\)
A = proportion of particles between 20 and 100 mesh size (assume 60 percent effective)
B = proportion of particles finer than 100 mesh size (assume 100 percent effective)
Equation 2
\(ENV=CCE\times\;x\left[\left(A\times0.6\right)+\left(B\times1\right)\right]\)
Or using equation #2 for the example above:
A = 65% (0.65), because 90% (finer than 20-mesh) minus 25% (finer than 100-mesh) equals 65% (between the 20- and 100-mesh sizes).
B = 25% (0.25)
\(ENV\ =0.80\ \times\;x\left[\left(0.65\times0.6\right)+\left(0.25\times1\right)\right]=0.80\times\;x0.64=0.512\)
Compared to standard agricultural lime, \(0.61\ \div0.512=1.19\). Thus, 1.19 tons of this material should be used for every 1 ton recommended on the soil test. Note the second equation suggests the material is less effective since it neglects contribution of the material in the 8- to 20-mesh size fraction. However, this is still preferable to the use of CCE alone, which completely neglects consideration of the fineness criterion.
Application and Incorporation
Lime is not very water soluble. Within one to three years, lime moves little in the soil and neutralizes acidity only in the zone where it is applied. To be most effective, lime must be uniformly spread and thoroughly incorporated. The poorest, but most common, method of application is by spinner spreader. Double spinner spreaders apply lime more uniformly than single spinner spreaders; however, both normally apply more product immediately behind the spreader than to its sides. In practice, rates are adjusted after checking the spreader pattern and making appropriate correction. If the application is not correct, strips of underlimed and overlimed soil could result, possibly reducing crop yields.
Special situations may occur in the coastal plain that will lead to overliming. First, if excessive lime falls along a relatively narrow path at the centerline of the spreader truck, the soil pH may increase somewhat above the desired level. Second, the delivered rate may be too high for sandy ridges that occur in certain fields. Third, too much lime may have been applied uniformly across the field. These three circumstances may elevate the pH to the extent that within a year or two of application, an "induced" manganese (Mn) deficiency will be seen. This is most likely to occur with soybeans, peanuts, and small grains, crops that are very sensitive to manganese deficiency.
Lime can be applied more evenly using full-width (box) or boom spreaders. Full-width spreaders allow lime to fall to the ground by gravity. The rate is determined by the size of the openings in the box and by ground speed. Boom spreaders use drag chains, augers, or air pressure to move lime out the booms and drop it on the ground. If adjusted properly both types of spreaders are vastly superior to the spinner type. The main limitations to their use are the high initial cost and more complex operation. Most growers will likely continue using spinner spreaders, but every attempt should be made to spread lime evenly.
The most commonly used lime incorporation tool is the disk. Its main limitation is that it incorporates lime only about half as deeply as the disk blades penetrate. Even with repeated passes, it does not incorporate lime well. Offset disks that throw the soil perform better. The best implement is a heavy-duty rotary tiller that mixes the soil throughout the root zone.
Bottom plowing immediately after spreading lime likely will bury the lime too deeply. If plowing, the best approach is to apply half the lime, then disk and bottom-plow, and then apply the other half and disk again. However, this process is costly and generally is not used.
Certain other tillage practices, such as bedding or middle busting, will help with lime incorporation in the long run. But chisel plowing is very ineffective. Although lime is applied on the surface to established pastures and lawns, it should be incorporated at establishment to reduce soil acidity.
Liming and Long-Term No-Till
Long-term no-till cultivation is becoming increasingly popular in North Carolina and obviously limits the ability to incorporate lime into the soil. A survey of no-till fields in North Carolina detected slightly higher soil pH at the surface with no-till management, a reflection of surface lime application. Nevertheless, producer experience suggests no inherent problem maintaining optimum soil pH with surface liming in long-term continuous no-till. It is critical, however, to correct soil acidity and other fertility problems, particularly with phosphorus, by thorough incorporation of lime and fertilizer prior to the adoption of no-till management. Research in Pennsylvania has documented that low soil pH problems can persist for several years following application of lime to the surface of no-till fields.
Maintenance of proper soil pH can increase your crop income. However, one size does not fit all. Varying rates of lime will be recommended, depending on what is judged the best pH for the particular soil class and crop combination. To learn your soil’s pH and its lime requirement, send a soil sample to Agronomic Division, North Carolina Department of Agriculture & Consumer Services.
References
Barber, S. A. 1984. “Liming materials and practices.” Chapter 4 (pp. 171- 209) in: F. Adams (ed.) Soil Acidity and Liming, 2nd edition. Agronomy Series No. 12, ASA, Madison, Wis.
Crozier, C. R., and D. H. Hardy. 2003. SoilFacts: Soil Acidity and Liming—Basic Information for Farmers and Gardeners. AG-439-51, North Carolina Cooperative Extension.
Crozier, C. R., G. C. Naderman, M. R. Tucker, and R. E. Sugg. 1999. “Nutrient and pH stratification with conventional and no-till management.” Commun Soil Sci Plant Anal 30(1&2):65–74.
Osmond, D. L., C. R. Crozier, and D. H. Hardy. 2002. SoilFacts: Careful Soil Sampling—The Key to Reliable Soil Test Information. AG-439-30, North Carolina Cooperative Extension.
Hardy, D. H., M. R. Tucker, and C. C. Carter. 2014. Crop Fertilization Based On North Carolina Soil Tests. Raleigh, N.C.: North Carolina Department of Agriculture, Agronomic Division. Agronomic Division Circular No. 1. 97 p.
NC Lime Law:
Acknowledgments
This publication is a revision of an earlier version. The authors would like to thank Carl Crozier for his earlier contributions.
Publication date: June 9, 2022
AG-439-50
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.