Introduction
The Albemarle-Pamlico Estuarine System (APES) in North Carolina, the second largest estuary in the United States, contains many species of submerged aquatic vegetation (SAV), which are vital to the ecological health of the system (Bartenfelder et al. 2022; Ferguson et al.1989; NCDEQ 2021a; Thayer et al. 1984). North Carolina ranks in the top three states for SAV abundance. The state’s coastal communities are reliant on the ecosystem services provided by a healthy APES environment, which include food, commercial and recreational fishing, and aesthetic amenities (Waite et al.1994).
Submerged aquatic vegetation includes seagrass meadows that grow in submerged, shallow aquatic locations (UNEP 2020). The primary species in North Carolina are eelgrass (Zostera marina), shoal grass (Halodule wrightii), and widgeon grass (Ruppia maritima), which reside in brackish water (Ferguson et al. 1989). Seagrass meadows are typically found in water less than two meters deep and are less abundant in the deeper, open water. These meadows serve many ecological functions. Seagrasses filter and store excess nutrients, which improves water quality and reduces seafood contamination. In addition, the meadows stabilize sediments, protect coastlines from erosion, and prevent sediment loss (UNEP 2020).
The combination of different SAV species in an estuary creates a unique environment that is abundant with life (Ferguson et al. 1989; Thayer et al. 1984). A survey conducted from 2006 to 2008 identified over 137,000 acres of SAV meadows in the APES (APNEP 2012a). Fish and shellfish species use SAV for protection, food, and as a nursery habitat. As many as 40,000 fish and 50 million small invertebrates are supported by one acre of seagrass (APNEP n.d). Species such as blue crab, red drum, and spotted sea trout rely on SAV nurseries and are economically important to North Carolina’s commercial and recreational fishing industries.
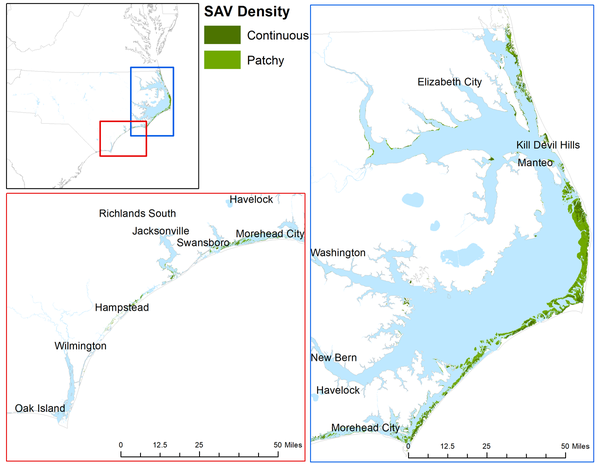
Figure 1 shows the maximum historical extent of SAV in the APES observed in mapping efforts from 1981-2021 using data from the NC Department of Environmental Quality (NC DEQ). Aerial surveys taken in high-salinity seagrass locations were used to produce the maps (Field et al. 2021). The SAV located in turbid water is more difficult to detect. Boat-based video and sonar have been used since 2010 to help determine the locations of low-salinity SAV.
In recent years, the APES experienced significant losses in SAV that have important economic consequences (Blandon et al. 2014; Field et al. 2021; Kenworthy 2020). According to research from the Albemarle-Pamlico National Estuarine Partnership (APNEP), the extent of seagrass in the APES decreased by 5.6% between 2006 and 2013 (NCDEQ 2021b). The loss has been attributed to nutrient and sediment pollution, which prevents light from reaching the submerged plants (NCDEQ 2021a).
We conducted a review of scientific literature on SAV in the APES in order to document its importance, identify key mechanisms of decline, and provide options for addressing this loss. Due to the dearth of information on low-salinity SAV in the APES, we focused almost exclusively on high-salinity seagrass and synthesized this information to explore the economic costs of SAV depletion.
Causes of SAV Decline
To understand and address the economic consequences of SAV loss, it is important to understand the causes of and contributors to SAV decline. Worldwide, nearly one-third of SAV has been lost since 1980 (Blandon et al. 2014). The immediate cause of SAV loss is light attenuation—the reduction of light available for photosynthesis as it travels through the water column.
As a submerged species, SAV relies on sufficient water clarity to allow for the transmission of sunlight. Decline in SAV abundance is strongly associated with a decrease in light availability from high turbidity that is caused by sediment pollution and eutrophication—the process by which high nutrient levels increase algal growth and reduce the oxygen available for aquatic life. Increased turbidity decreases the maximum growing depth of SAV. In the APES, limited light availability inhibits SAV growth to depths of 0.87 m to 2 m (Biber et al. 2008). Turbidity is higher in spring and summer due to increased algal growth, which coincides with the SAV growing season. Other factors that contribute to light attenuation include suspended particulate matter, colored dissolved organic matter, increased algal growth in the water column, and shading from epiphytic growth, which involves smaller plants that grow on the surface of SAV (Carter et al. 1990; Harvey et al. 2019; Kemp et al. 2004).
Table 1 shows the factors involved in SAV decline identified in the literature. The level of importance was determined by the severity of each factor in causing SAV declines in other locations, and the prevalence in North Carolina. Our understanding was based on the literature and research efforts in North Carolina, which were synthesized to determine the role of each factor in causing SAV decline in the APES.
Sediment and Eutrophication
Sediment pollution, in which particles are carried from a physical location and washed into bodies of water, occurs from agricultural runoff, logging activities, runoff from construction and urban sites, and shoreline erosion. Sediment can also be created by storms, boat traffic, jet skis, and other causes (EPA 2006). As the total sediment that is suspended in the water column increases, the light available for SAV decreases. The SAV populations that are closest to the mainland are most susceptible to human-induced sediment pollution, although there is significant sediment throughout the estuary.
Another cause of sediment pollution in APES is dredging that uses sand mining and construction activities to deepen navigation channels (Erftemeijer et al. 2006). In the Currituck Sound, a northern region of the APES, SAV coverage declined after the construction, widening, and deepening of the Albemarle and Chesapeake Canal, as well as dredging in the North Landing River in 1918 (Carter et al. 1994). Near Morehead City in North Carolina, significant SAV loss occurred after nearby dredging of access channels (Stallings et al. 2014). In addition to increasing sediment pollution, dredging can cause the physical uprooting and burial of SAV, as well as changes in bathymetry (the depth of the estuary), which can decrease the amount of habitat suitable for recruitment, the process in which small fish transition to an older stage (Erftemeijer et al. 2006).
Pollution can also change the composition of the sediments in which SAV grows. The ideal growing conditions for most SAV species are sediments where there is less than 20% to 30% silt and clay by weight and less than 5% organic matter (Kemp et al. 2004). Silt and clay describe the particle size of the sediment. Clay is classified as less than 0.002 mm in diameter, while silt is between 0.002 mm and 0.05 mm.
In North Carolina, SAV decline near rivers has been especially severe, with a 97% loss along the Pamlico River and similar trends seen along the Neuse River (Kenworthy 2020). This change is believed to be due to alterations in sediment composition and nutrient concentrations. Most of the sediment that enters the APES through rivers is high in clay, silt, and organic matter (Wells et al. 1989). River mouths are usually more shallow, and therefore more suitable for SAV than the surrounding estuary. Habitat degradation in these locations tends to be particularly damaging to SAV populations (Biber et al. 2008; Wells et al. 1989).
Eutrophication, the other key cause of SAV decline, occurs when excessive organic matter promotes high rates of macroalgal and phytoplankton growth. The algae cover the surface of the water, prevent light penetration, and reduce oxygen production from photosynthesis. Decaying algae decreases the amount of dissolved oxygen in the water, which leads to fish kills. As of 2001, roughly 65% of the United States estuarine surface area displayed moderate to high eutrophic conditions. The highest eutrophic areas have been documented in the Mid-Atlantic and Gulf Coast regions (Clement et al. 2001).
Other Causes
While there has been discussion about the effects of herbicide pollution, epiphytes, sulfide toxicity, disease, and climate change on SAV health, these factors alone are unlikely to be responsible for extensive declines in SAV. Instead, these factors compound the existing damage from eutrophication and sediment pollution.
Herbicide Concentrations
Herbicides have been indirectly linked to SAV degradation. Studies that used atrazine as a pre-exposed herbicide on Vallisneria americana Michx, an SAV species also known as wild celery, showed an impairment of photosynthetic activity along with lower values of dissolved oxygen (Dantin et al. 2009). This toxic environment led to a decrease in epiphyte grazers, which feed on the epiphytic algae growing on seagrasses and naturally control epiphyte abundance and shading. Thus, epiphyte populations increase continuously and reduce seagrass growth (Short et al. 1995). Herbicides such as atrazine, which has been found at high levels in the APES, have the greatest negative effect on seagrasses with higher growth rates (Powell et al. 2017). In 2000, levels of predominant herbicides in the transition zone of the Tar River and the APES exceeded the documented US Environmental Protection Agency (EPA) vascular plant life thresholds. For example, the concentration of the herbicide Alachlor has been measured at 6,100 ng/L, which was more than double the 2,300 ng/L benchmark for vascular plant survival (Powell et al. 2017).
Epiphytes
Epiphytes are plants that grow on the surface of other vascular plants. Under normal conditions, SAV species such as eelgrass have a symbiotic relationship with epiphytes, which benefit from the habitat and access to sunlight that is provided by seagrasses. In return, the algae exchange nutrients with the seagrass and can contribute to roughly 18% of areal eelgrass productivity (Penhale 1977). High epiphyte loads, however, create shading on the SAV, which prevents light from reaching its leaves due to the physical epiphytic barrier. Excess nutrient loading and eutrophic conditions increase the growth of epiphytes, which leads to a parasitic relationship (Carter et al. 1990; Kemp et al. 2004).
Epiphyte accumulation creates a thick crust on the leaves of seagrasses, which then shades the leaves (Sand-Jensen 1977). Sediment can easily attach itself to this thick coating, which further reduces light and seagrass photosynthesis (Montfrans et al. 1984). Epiphyte-crowded SAV was found to experience 31% less photosynthetic productivity at optimum light conditions, and this effect progressively worsens at lower light conditions (Sand-Jensen 1977). Light attenuation caused by epiphytes also negatively affects sexual reproduction (Montfrans et al. 1984).
Research on epiphytes and SAV productivity is difficult to evaluate because of issues with properly removing epiphytes for experimentation. Removal attempts often cause damage to the seagrass leaves, which obscures the exact values for photosynthesis productivity needed in the studies (Montfrans et al. 1984; Sand-Jensen 1977).
Sulfide Toxicity
Clay also affects SAV through its capacity to bind to humus, the decomposed organic matter content of soil, to form clay-organic complexes. High influxes of clay can introduce large amounts of organic matter into the system, which is linked to sulfide toxicity. Sulfide, the end-state product of sulfate reduction, is a phytotoxic compound that affects plant growth, fitness, and ecosystem function (Havill et al. 1985).
The severity of sulfide toxicity depends on the species of plant, although high-sulfide conditions are generally damaging to SAV. In coastal salt marshes, for example, plant height and biomass production were inhibited at concentrations of sulfide as low as 1.0 mM (Bradley et al. 1989). It is possible that the influxes in organic matter are promoting eutrophic conditions and increasing sulfide toxicity simultaneously. In order to fully understand the relative contribution of sulfide toxicity to SAV decline, more research is needed to identify species-specific tolerance levels and the impact of varying sediment compositions in the APES.
Disease
There are numerous pathogens that can damage SAV. A disease of particular concern is wasting disease, which has been found in some eelgrass populations in the southern Core Sound, Back Sound, and Bogue Sound in North Carolina (South Atlantic Fishery Management Council 1998). If wasting disease or other pathogens are a key cause of SAV decline in the APES is currently uncertain, although it is well-documented that herbicides and eutrophication are connected to disease susceptibility. Phenolic acids, a natural defense mechanism against pathogens, decrease during high nitrate conditions or decreased light availability (Vergeer et al. 1997). Hughes et al. (2017) found that eelgrass shoots grown in high nitrate concentrations were much more susceptible to infection by Labyrinthula zosterae due to a decrease in the structural integrity of SAV cell walls.
Climate Change
The conservation of SAV worldwide faces challenges from climate change, which may result in local areas of destruction, changes in community composition, and a loss of genetic diversity (Chefaoui et al. 2018; Micheli et al. 2008). Increased tidal height and a rise in sea level decrease light availability and lower photosynthetic rates, which may further reduce habitat that is limited by poor water quality.
Altered salinity from saltwater intrusion and increased temperature may work together with changes in sea level and tidal height to decrease SAV abundance (Orth et al. 2010; Sunny 2017). The effects of increased water temperature are particularly concerning given the seagrass community present in the APES, which includes both temperate and tropical species at the edges of their ranges. These populations may be particularly vulnerable to climate change and experience long-term shifts in biomass and areal extent, which then decrease overall meadow function (Bartenfelder et al. 2022). Changes in the seagrass community composition will likely have cascading effects that, regardless of species replacement, alter faunal assemblages, as documented in Bogue Sound, North Carolina (Micheli et al. 2008).
Estimating the Benefits of SAV
Understanding the relative importance of the factors implicated in the decline of SAV is particularly important given the vital role that SAV meadows play in supporting the North Carolina economy through key ecosystem services. In addition to filtering, storing excess nutrients, and reducing seafood contamination, SAV meadows are nurseries for economically important fish species. The SAV meadows also improve water quality, sequester carbon, and protect coastlines from erosion by preventing sediment loss.
Between 2006 and 2013, SAV loss in the APES was estimated to be 5.6%, or 0.8% per year (Field et al. 2021). In the next section, we report the analysis of the economic losses due to SAV decline based on Sutherland et al. (2021). These costs are estimated for four different scenarios of SAV decline over ten years: 5%, 15%, 25%, and 50% total loss, with ranges from slightly below previous estimates of annual losses to accelerated loss above current levels. The loss estimates are limited to the next ten years, because this is the period in which policy makers typically develop coastal policy.
The Fishing Industry
In North Carolina, many species—including blue crab, gray trout, red drum, spotted seatrout, mullet, spot, pinfish, gag grouper, white grunt, silver perch, summer flounder, southern flounder, and hard-shell clams—use SAV as nursery grounds. Blue crab is the highest value commercial fishing species in North Carolina (Edwards et al. 2021, Greene 2006). For blue crab, SAV increases density by supplying a protective habitat with access to food, and provides a surface for larval and post-larval stage blue crabs to settle (Tran 2003). Other fish and shellfish species reap similar benefits, especially those that are entirely dependent on SAV for recruitment, in which the very young can survive to become more mature fish (South Atlantic Fishery Management Council 1998). Sutherland et al. (2021) estimate that declining SAV abundance would result in large losses to commercial and recreational fisheries. The report offered a range of SAV loss scenarios in APES over the next decade with estimated losses to blue crab fishery of up to $6.7 million, and losses up to $5 million to the spotted sea trout and red drum recreational fisheries.
Water Quality and Home Values
Buyers of coastal property are less willing to pay for locations that contain polluted waters or those without recreational fishing (Nicholls and Crompton 2018). Water quality is directly affected by SAV because SAV stabilizes and traps sediments, recycles nutrients, and increases dissolved oxygen levels. This process can increase the clarity of the water, improve the visual aesthetics of the location, and the abundance of aquatic species (Nicholls and Crompton 2018; UNEP 2020). In addition, SAV also provides value for property owners through its role in mitigating coastal erosion. The improvements to water quality, erosion control, and increased recreation opportunities afforded by SAV have positive effects on home values along estuarine shorelines. Declining SAV abundance over the next decade is estimated to result in lost property values that range from $2 to $23 million in the APES, depending on the level of SAV depletion (Sutherland et al. 2021).
Carbon Sequestration
Carbon sequestration is a vital ecosystem service through which organic and inorganic carbon emissions are absorbed and stored, and which modulates or prevents the release of the greenhouse gas (carbon dioxide) back into the atmosphere where it contributes to climate change. The canopies of SAV capture carbon particles in the water column by filtration, reduce the re-suspension of organic carbon particles, and bury them within the sediment (Kennedy et al. 2010). Sediments that are vegetated with seagrasses hold twice the amount of carbon as non-vegetated locations, while eelgrass meadows have been shown to hold triple the amount (McGlathery, et al. 2012). It is estimated that the value of carbon stored in APES seagrass meadows ranges from $163 to $419 million, while the value of lost carbon sequestration over the next decade will range from $5.6 to $55.6 million, depending on the level of SAV decline (Sutherland et al. 2021).
Erosion Control
North Carolina has historically experienced an erosion rate of 1.6 m per year, which is typically caused by wave action and human activities (Eulie et al. 2017). The control of coastal erosion by SAV occurs by its ability to reduce current velocities, stabilize sediment (Duarte et al. 2013; Koch et al. 2006; Ondiviela et al. 2014), and attenuate wave energy (Koch et al. 2006). The root systems of SAV stabilize sediment, while SAV also lowers the current velocity by decreasing the size and strength of the waves that reach the shoreline (Barbier et al. 2011; Koch et al. 2006). In addition, SAV may also play an important role in the formation and stabilization of coastal dunes (Hemminga and Nieuwenhuize 1990).
North Carolina SAV Policy
In 2016, the NC Department of Environmental Quality (NCDEQ) created the North Carolina Coastal Habitat Protection Plan (CHPP) through the Fisheries Reform Act of 1997. Initially, SAV played a brief role in this conservation plan until its recent amendment in 2021, which placed greater emphasis on SAV protection in Chapter 4. The CHPP amendment has increased water quality monitoring that uses new SAV benchmarks for light penetration levels and chlorophyll a concentration. However, long-term monitoring requires consistent funding, which has yet to be obtained (NCDEQ 2021a).
The Albemarle-Pamlico National Estuary Partnership (APNEP) created a ten-year Comprehensive Conservation and Management Plan (CCMP) in 2012. Guidelines to conserve SAV are identified as a component of protecting and managing significant habitat. The restoration of SAV habitat is listed under facilitating restoration projects in the plan (APNEP 2012b). Mapping of SAV extent, species composition and abundance, and changes in extent have been conducted by APNEP under the CCMP (APNEP 2012b).
A major difference between the CCMP and the CHPP is the adoption of a more holistic ecological approach. The CCMP’s watershed perspective focuses on the sources of water quality impairment. This view recognizes inland sources of nutrients and sediment as key drivers of SAV decline.
Restoring SAV is quite costly relative to preserving current SAV populations. In the Chesapeake Bay Program, the replanting costs alone ranged from $5,000 to $15,000 per acre (Chesapeake Bay Program 2006). The total price varies greatly based on environmental conditions, restoration methods, labor, and the loss of transplanted material or pre-existing beds (Christensen et al. 2004). Bayraktarov et al. (2016) report that the average total cost of SAV restoration projects is roughly $50,000 per acre and the median survival rate for restored SAV one to two years after project completion is only 38%.
Conclusions
Declines in SAV coverage and density in the APES are problematic due to the important economic benefits from this habitat. A review of the current literature suggests that light attenuation due to eutrophication and sediment pollution is the key cause. Sources of eutrophication and sediment in the APES are primarily human activities that lead to nutrient and sediment pollution. Other contributing factors include herbicide loading, epiphyte prevalence, sulfide toxicity, and SAV diseases. Although restoration is an option for replacing lost SAV acreage, policies that reduce sediment and nutrient pollution offer more cost effective approaches for preserving the existing SAV beds.
There are important gaps in our understanding of SAV decline, particularly those related to the key drivers of decline in the APES. In general, there is little information about the specific sources of problematic sediment and nutrient loads. Further research is needed to better understand the distribution, extent, and abundance of low-salinity SAV, as well as the relative importance of different stressors in its decline. While SAV near river mouths is particularly vulnerable, further research is required to establish other locations that are at elevated risk of decline, as well as to assess the vulnerability of individual species.
It is certain that SAV is vital to North Carolina’s estuarine health and coastal economy. Sound policy decisions, including the funding of conservation, restoration, and research efforts, are necessary to safeguard this resource now and into the future.
References
Albemarle-Pamlico National Estuary Partnership (APNEP). 2012a. 2012 Albemarle-Pamlico Ecosystem Assessment. Raleigh: Albemarle-Pamlico National Estuary Partnership. ↵
Albemarle-Pamlico National Estuary Partnership (APNEP). 2012b. Comprehensive Conservation and Management Plan, 2012 - 2022. Raleigh: Albemarle-Pamlico National Estuary Partnership. ↵
Albemarle-Pamlico National Estuary Partnership (APNEP). n.d. “What is the Albemarle-Pamlico Region?” Raleigh: Albemarle-Pamlico National Estuary Partnership.↵
Barbier, Edward B., Sally D. Hacker, Chris Kennedy, Evamaria W. Koch, Adrian C. Stier, and Brian R. Silliman. 2011. “The Value of Estuarine and Coastal Ecosystem Services.” Ecological Monographs 81, no. 2: 179-93. ↵
Bartenfelder Amy, William J. Kenworthy, Brandon Puckett, Charles Deaton, and Jesse C. Jarvis. 2022. “The Abundance and Persistence of Temperate and Tropical Seagrasses at Their Edge-of-Range in the Western Atlantic Ocean.” Frontiers in Marine Science 9, no. 917237:1-21. ↵
Bayraktarov, Elisa, Megan I. Saunders, Sabah Abdullah, Morena Mills, Jutta Beher, Hugh P. Possingham, Peter J. Mumby, and Catherine E Lovelock. 2016. “The Cost and Feasibility of Marine Coastal Restoration.” Ecological Applications 26, no. 4: 1055-1074. ↵
Biber, Patrick D., Charles L. Gallegos, and W. Judson Kenworthy. 2008. “Calibration of a Bio-optical Model in the North River, North Carolina (Albemarle–Pamlico Sound): A Tool to Evaluate Water Quality Impacts on Seagrasses.” Estuaries and Coasts 31:177–191. ↵
Blandon, Abigayil, and Philine S.E. zu Ermgassen. 2014. “Quantitative Estimate of Commercial Fish Enhancement by Seagrass Habitat in Southern Australia.” Estuarine, Coastal, and Shelf Science 141, no. 2014: 1–8. ↵
Bradley, Paul M., and E. Lloyd Dunn. 1989. “Effects of Sulfide on the Growth of Three Salt Marsh Halophytes of the Southeastern United States.” American Journal of Botany 76, no.12: 1707–1713. ↵
Carter, Virginia, and Nancy B. Rybicki. 1990. “Light Attenuation and Submersed Macrophyte Distribution in the Tidal Potomac River and Estuary.” Estuaries and Coasts 13, no. 4: 441-452. ↵
Carter, Virginia, and Nancy B. Rybicki. 1994. “Invasions and Declines of Submersed Macrophytes in the Tidal Potomac River and Estuary, the Currituck Sound-Back Bay System, and the Pamlico River Estuary.” Lake and Reservoir Management 10, no.1: 39–48. ↵
Chefaoui, Rosa M., Carlos M. Duarte, and Ester A. Serrão. 2018. “Dramatic Loss of Seagrass Habitat Under Projected Climate Change In The Mediterranean Sea.” Global Change Biology 24, no. 10: 4919 – 4928. ↵
Chesapeake Bay Program. 2006. Best Management Practices for Sediment Control and Water Clarity Enhancement. Annapolis: Chesapeake Bay Program. ↵
Christensen, Peter Bondo, Elena Diaz Almela, and Onno Diekmann. 2004. “Can Transplanting Accelerate the Recovery of Seagrasses?” European Seagrasses: An Introduction to Monitoring and Management Copenhagen: The M & MS Project. ↵
Clement, Chris, S. B Bricker, and D.E. Pirhalla. 2001. “Eutrophic Conditions in Estuarine Waters.” NOAA’s State of the Coast Report. Silver Spring: National Oceanic and Atmospheric Administration. ↵
Dantin, Darrin D., Ronald G. Boustany, Steven J. Jordan, Michael A. Lewis, Rebecca F. Moss, and Thoma C. Michot. 2009. “Effects of Nutrient Pre-Exposure on Atrazine Toxicity to Vallisneria americana Michx. (Wild Celery).” Archives of Environmental Contamination and Toxicology 58, no. 3: 622–630. ↵
Duarte, Carlos, M., Iňigo J. Losada, Iris E. Hendriks, Inéz Mazarrasa, and Núria Marbà. 2013. “The Role of Coastal Plant Communities for Climate Change Mitigation and Adaptation.” Nature Climate Change 3, no.11: 961-968. ↵
Edwards, Eric C., Sara A. Sutherland, Chris Dumas, Andrew Hitchens, and Yetunde Oshagbemi. 2021. “North Carolina’s Wild Caught Fishing Industry Economic Impact Assessment.” N.C. Sea Grant Report. Silver Spring: National Sea Grant College Program. ↵
Environmental Protection Agency (EPA). 2006. “Chapter 15: Turbidity and Total Solids.” Voluntary Estuary Monitoring: A Methods Manual. Washington: EPA. ↵
Erftemeijer, Paul L.A., and Roy Robin Lewis III. 2006. “Environmental Impacts of Dredging on Seagrasses: A Review.” Marine Pollution Bulletin 52, no.12: 1553–1572. ↵
Eulie, Devon O., J.P. Walsh, D.R. Corbett, and Ryan P. Mulligan. 2017. “Temporal and Spatial Dynamics of Estuarine Shoreline Change in The Albemarle-Pamlico Estuarine System, North Carolina, USA.” Estuaries and Coasts 40, no. 3: 741–757. ↵
Ferguson, R. L., J. A. Rivera, and L. L. Wood. 1989. “Submerged Aquatic Vegetation in the Albemarle-Pamlico Estuarine System.” Albemarle-Pamlico Estuarine Study. Beaufort: National Marine Fisheries Service, North Carolina Department of Environment, Health, and Natural Resources, and the Environmental Protection Agency. ↵
Field, Don, Judd Kenworthy, and Dean Carpenter. 2021. “Metric Report: Extent of Submerged Aquatic Vegetation in High-Salinity Estuarine Waters.” Raleigh: Albemarle-Pamlico National Estuary Partnership. ↵
Greene, Emily. 2006. “Managing the North Carolina Blue Crab Fishery: Engaging Fishermen in the Analysis of Soft and Peeler Crab Regulations.” Durham: Duke University, Master’s Thesis. ↵
Harvey, E. Therese, Jakob Walve, Agnette Andersson, Bengt Karlson, and Susanne Kratzer. 2019. “The Effect of Optical Properties on Secchi Depth and Implications for Eutrophication Management.” Frontiers in Marine Science 5: 496. ↵
Havill, D.C., A. Ingold, and J. Pearson. 1985. “Sulfide Tolerance in Coastal Halophytes.” Vegetatio 62, no. 103: 279–285. ↵
Hemminga, M.A., and J. Nieuwenhuize. 1990. “Seagrass Wrack-Induced Dune Formation on a Tropical Coast (Banc d'Arguin, Mauritania).” Estuarine Coastal and Shelf Science 31, no. 4: 499-502. ↵
Hughes, R. G., M. Potouroglou, Z. Ziauddin, and J.C. Nicholls. 2017. “Seagrass Wasting Disease: Nitrate Enrichment and Exposure to a Herbicide (Diuron) Increases Susceptibility of Zostera Marina to Infection.” Marine Pollution Bulletin. 134, September 2018: 96-98. ↵
Kemp, W. Michael, Richard Batiuk, Richard Bartleson, Peter Bergstrom, Virginia Carter, Charles L. Gallegos, William Hunley, Lee Carrh, et al. 2004. “Habitat Requirements for Submerged Aquatic Vegetation in the Chesapeake Bay: Water Quality, Light Regime, and Physical Chemical Factors.” Estuaries and Coasts 23, no. 3: 363-377. ↵
Kennedy, Hillary, Jeff Beggins, Carlos M. Duarte, James W. Fourqurean, Marianne Holmer, Núria Marbă, and Jack J. Middelburg. 2010. “Seagrass Sediments as a Global Carbon Sink: Isotopic Constraints.” Global Biogeochemical Cycles 24, no. GB4026:1–8. ↵
Kenworthy, Jud. 2020. “The Extent and Status of Submerged Aquatic Vegetation in North Carolina.” ↵
Koch, Evamaria W., Larry P. Sanford, Shi-Nan Chen, Deborah J. Shafer, and Jane M. Smith. 2006. Waves in Seagrass Systems: Review and Technical Recommendations. Washington: Army Corps of Engineers. ↵
McGlathery, Karen J., Laura Reynolds, Luke W. Cole, Robert J. Orth, Scott R. Marion, and Arthur Schwarzschild. 2012. Recovery Trajectories During State Change From Bare Sediment to Eelgrass Dominance. Marine Ecology Progress Series 448: 209-221. ↵
Micheli, Fiorenza, Melanie J. Bishop, Charles H. Peterson, and José A. Rivera. 2008. “Alteration of seagrass species composition and function over two decades.” Ecological Monographs 78 (20): 225-244. ↵
Montfrans, Jacques V., Richard L. Wetzel, and Robert J. Orth. 1984. “Epiphyte-Grazer Relationships in Seagrass Meadows: Consequences for Seagrass Growth and Production.” Estuaries 7, no.4A: 289–309. ↵
Nicholls, Sarah, and John Crompton. 2018. “A Comprehensive Review of the Evidence of the Impact of Surface Water Quality on Property Values.” Sustainability 10, no. 2: 1–30. ↵
North Carolina Department of Environmental Quality (NCDEQ). 2016. North Carolina Coastal Habitat Protection Plan. ↵
North Carolina Department of Environmental Quality (NCDEQ). 2021a. North Carolina Coastal Habitat Protection Plan 2021 Amendment. ↵
North Carolina Department of Environmental Quality (NCDEQ). 2021b. “North Carolina’s Seagrass Habitat is Declining, State-Federal Partnership Data Show.” ↵
Ondiviela, Barbara, I.J. Losada, Javier L. Lara, Maria Maza, Christina Galván, Tjeerd J. Bouma, and Jim van Belzen. 2014. “The Role of Seagrasses in Coastal Protection in a Changing Climate.” Coastal Engineering 87, May 2014: 158-168. ↵
Orth, Robert J., Michael R. Williams, Scott R. Marion, David J. Wilcox, Tim J. Carruthers, Kenneth A. Moore, W. Michael Kemp, et al. 2010. “Long-Term Trends in Submersed Aquatic Vegetation (SAV) in Chesapeake Bay, USA, Related to Water Quality.” Estuaries and Coasts 33, no. 5: 1144–1163. ↵
Penhale, Polly A, Walker O. Smith Jr. 1977. “Excretion of dissolved organic carbon by eelgrass (Zostera marina) and its epiphytes.” Limnology and Oceanography 22, no. 3: 400–407. ↵
Powell, Kelly W., W. Gregory Cope, Catherine E. LePrevost, Tom Augspurger, Annette M. McCarthy, and Damien Shea. 2017. “A Retrospective Analysis of Agricultural Herbicides in Surface Water Reveals Risk Plausibility for Declines in Submerged Aquatic Vegetation.” Toxics 5, no. 3: 21. ↵
Sand-Jensen, Kaj. 1977. “Effect of Epiphytes on Eelgrass Photosynthesis.” Aquatic Botany 3, 1977: 55-63. ↵
Short, Frederick T., David M. Burdick, and James E. Kaldy III. 1995. “Mesocosm Experiments Quantify the Effects of Eutrophication on Eelgrass, Zostera marina.” Limnology and Oceanography 40, no.4: 740–749. ↵
South Atlantic Fishery Management Council (SAFMC). 1998. Final Habitat Plan for the South Atlantic Region: Essential Fish Habitat Requirements for Fishery Management Plans of the South Atlantic Fishery Management Council. Charleston: South Atlantic Fishery Management Council. ↵
Stallings, Kevin, Robert J. Richardson, Brett Hartis, and Steve T. Hoyle. 2014. Influence of Shading on Submerged Aquatic Vegetation from Bridge Structures. Raleigh: North Carolina Department of Transportation. ↵
Sunny, Atiqur R. 2017. “A Review on Effect of Global Climate Change on Seaweed and Seagrass.” International Journal of Fisheries and Aquatic Studies. 5, no. 6: 19-22. ↵
Sutherland, Sara, Roger Von Haefen, Jie Cao, and David Eggleston. 2021. Economic Valuation of Submerged Aquatic Vegetation in the Albermarle-Pamlico Estuary. Raleigh: Albemarle-Pamlico National Estuary Partnership. ↵
Thayer, Gordon W., W. Judson Kenworthy, and Mark S. Fonseca. 1984. The Ecology of Eelgrass Meadows of the Atlantic Coast: A Community Profile. Washington: Fish and Wildlife Service, U.S. Department of the Interior. ↵
Thompson, Austin. 2018. “Growing Economies and Shrinking Coastlines: Financing Wider Beaches.” Chapel Hill: UNC Environmental Finance Center. ↵
Tran, Lynn. 2003. “North Carolina’s Blue Crab Dilemma.” Raleigh: North Carolina State University. ↵
United Nations Environment Programme (UNEP). 2020. Out of the Blue: The Value of Seagrasses to the Environment and to People. Nairobi: UNEP. ↵
Vergeer, Luc. H. T., and Akin Develi. 1997. “Phenolic Acids in Healthy and Infected Leaves of Zostera marina and Their Growth-Limiting Properties Towards Labyrinthula zosterae.” Aquatic Botany 58, no.1: 65-72. ↵
Waite, Randall, and APNEP Staff. 1994. Comprehensive Conservation and Management Plan - Guide to Environmental & Economic Stewardship in the Albemarle-Pamlico Region. Raleigh: NC Department of Environment, Health, and Natural Resources. ↵
Wells, John T., and Seok-Yun Kim. 1989. “Sedimentation in the Albemarle-Pamlico Lagoonal System: Synthesis and Hypotheses.” Marine Geology 88, no. 3-4: 263–284. ↵
Publication date: July 17, 2023
AG-949
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.