Introduction
Blue mold of tobacco is caused by Peronospora hyoscyami f.sp. tabacina, an oomycete that is highly destructive to tobacco seed beds, transplants, and production fields in the humid farming zones of the southeastern and eastern United States, Canada, and countries bordering the Caribbean basin. The disease was first reported in the United States in 1921 in Florida and Georgia, and reappeared in the same region in 1931, spreading north into North Carolina, Virginia and Maryland. In subsequent years, the disease spread farther into the burley tobacco producing areas of Kentucky and Tennessee. By the 1960s, P. tabacina had been identified in Mexico, Canada, South America, the Caribbean, the Middle East, and many European nations. Currently, blue mold has been sporatically found in burley tobacco producing areas, but poses a low risk to flue-cured tobacco. Blue mold has not been found in North Carolina since 2017.
Host Range
Blue mold affects several Nicotiana species, including all cultivated tobacco types grown in North Carolina and several wild types, such as Nicotiana benthemiana. Where it is capable of overwintering, the pathogen survives on wild tobacco and can affect cultivated tobacco when conditions are present for spore dispersal and germination.
Pathogen
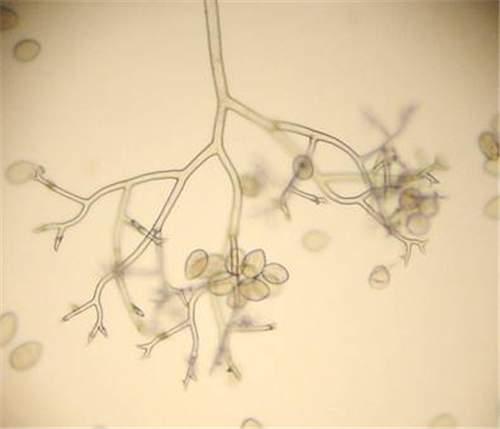
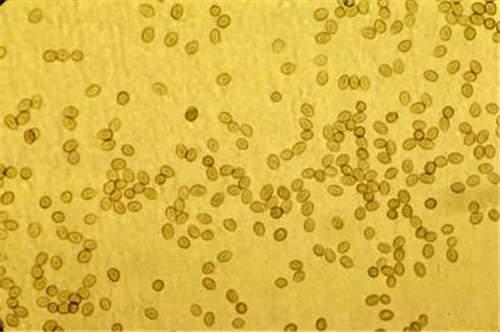
Tobacco blue mold is a downy mildew disease caused by the oomycete, Peronospora hyoscyami f.sp. tabacina. Peronospora tabacina is an 'obligate biotroph parasite', which means that it requires a living host to grow and survive. The asexual spores (sporangia) of Peronospora tabacina (Figure 1 and Figure 2) do not produce zoospores, so infection occurs via direct germination of these sporangia. Oospores (sexual overwintering spores) may be produced when two mating types merge in the field, but their role in the disease cycle is poorly understood.
Symptoms and Signs
In general, burley tobacco is much more susceptible to blue mold than flue-cured varieties. Once established, blue mold is fairly easy to identify, although symptoms vary with plant age.
Greenhouse
In the greenhouse, small patches of dead or dying seedlings provides evidence that the disease may be present. Foliar lesions appear yellow, grey, or bluish characteristic of blue mold.The upper surfaces of infected leaves will remain almost normal in appearance for 1-2 days before the plants begin to die and turn light brown. Diseased leaves often become twisted so that the lower surfaces turn upward.
In-Field
Blue mold can affect plants in the field throughout the growing season, and scouting for disease is important for timely management decisions. Single or groups of yellow spots (lesions) appear on the older, shaded leaves (Figure 3 and Figure 4). Often the spots grow together to form light brown, necrotic (dead) areas. Leaves become puckered and distorted, large portions disintegrate, and the entire leaf may fall apart (Figure 5). Under continuous favorable weather conditions, blue mold can destroy all leaves at any growth stage. Lesions may occur on buds, flowers, and capsules. In its early stages, blue mold can easily be confused with other leaf spots, cold injury, malnutrition, or damping-off; however, the presence of the characteristic gray-blue spores on the bottom of leaves (Figure 6) distinguishes it from other problems.
In severe situations, blue mold may also cause systemic stem infections, resulting in partial or overall stunting of the plant, with narrow, mottled leaves (Figure 7). Discoloration (brown streaks) can be found inside these stems. The plants often lodge or snap off if systemic infections occur near the base of weakened stems.
Disease Cycle and Conditions Favorable for Disease
The pathogen is not known to overwinter in North Carolina, and it is assumed that inoculum is introduced via wind blown spores. Sporangia can be dispersed thousands of kilometers by weather events and are the primary source of inoculum for epidemics. Likely sources of yearly blue mold epidemics are windblown spores from tobacco crops in Mexico and the Caribbean that move northward, or from wild tobacco in the southwestern US. It is unclear whether the pathogen is capable of overwintering in infected debris and the role of oospores in disease is not clearly understood. Another common way blue mold spreads is by the distribution of infected transplants. In some cases, transplants that appear healthy may actually be infected. Transplants that are obtained from other regions may harbor the pathogen and introduce the disease to the region.
Once blue mold is present, its development depends on weather conditions. Spores require wet leaves for germination and infection. Spores land on the damp surface of the tobacco plant (typically during morning hours) and then begin to germinate, sometimes beginning infection in a little as 2-4 hours. The pathogen produces structures to enter and colonize the plant tissue and then grows and consumes the nutrient from the plant. The pathogen can invade the leaf veins, petioles, and vessels of the stem. Sporulation begins may begin soon after visual symptoms are observed, and optimal conditions for fungal growth occurs when the temperature is between 60-75°F and humidity is greater than 95%. Under favorable conditions, the next generation of spores is produced within 7-10 days after initial infection. Spores produced are released during the morning hours when temperature rises and humidity decreases.
Peronospora tabacina is dependent on climate for disease development. It requires cool, wet, and cloudy weather to produce spores. Conversely, when the climate is sunny, hot, and dry, the pathogen survives poorly due to the dependence on moisture and high sensitivity to UV light. Therefore, when conditions are persistently cool and cloudy, disease epidemics may become severe and halted when conditions become hot and dry.
Management
Because the pathogen is highly dependent on a cool, humid environment in order to reproduce, one of the best strategies to prevent the development of an epidemic is to keep the growing area as thoroughly drained as possible. Maintaining appropriate plant spacing and nutrition to prevent excess growth and humidity in the canopy may also reduce disease.
Applying fungicides when the pathogen is most vulnerable is a critical aspect of managing blue mold. Several fungicides are labeled for blue mold management and can be found in the Flue-Cured Tobacco Guide or the North Carolina Agricultural Chemicals Manual.
If blue mold is suspected in a field, the best practice would be to call your local extension agent for confirmation. Plant samples should also be submitted to the Plant Disease and Insect Clinic.
Other Useful Resources
The NC State University Plant Disease and Insect Clinic provides diagnostic and control recommendations.
The NC State Extension Plant Pathology portal provides information on crop disease management.
The North Carolina Agricultural Chemicals Manual provides pesticide information for common diseases of North Carolina crops. Recommendations do not replace those described on the pesticide label, the label must be followed.
Your N.C. Cooperative Extension agent.
Publication date: Oct. 7, 2020
Reviewed/Revised: Dec. 11, 2024
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.
NC Cooperative Extension prohíbe la discriminación por raza, color, nacionalidad, edad, sexo (incluyendo el embarazo), discapacidad, religión, orientación sexual, identidad de género, información genética, afiliación política, y estatus de veteran.
The use of brand names in this publication does not imply endorsement by NC State University or N.C. A&T State University of the products or services named nor discrimination against similar products or services not mentioned.
Recommendations for the use of agricultural chemicals are included in this publication as a convenience to the reader. The use of brand names and any mention or listing of commercial products or services in this publication does not imply endorsement by NC State University or N.C. A&T State University nor discrimination against similar products or services not mentioned. Individuals who use agricultural chemicals are responsible for ensuring that the intended use complies with current regulations and conforms to the product label. Be sure to obtain current information about usage regulations and examine a current product label before applying any chemical. For assistance, contact your local N.C. Cooperative Extension county center.
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.