Introduction
Weed interference can result in large yield losses in sweetpotato (Seem et al. 2003; Meyers et al. 2010; Meyers and Shankle 2016). Palmer amaranth (Amaranthus palmeri) is the most common and most troublesome weed in North Carolina sweetpotato, followed by yellow nutsedge (Cyperus esculentus) (Webster 2010). It is an aggressive weed that is highly competitive with peanut, soybean, cotton, and sweetpotato (Burke et al. 2007; Chandi et al. 2012; Meyers et al. 2010; Morgan et al. 2001; Smith et al. 2020). Most Palmer amaranth populations in North Carolina are resistant to glyphosate and acetolactate synthase (ALS) inhibiting herbicides. It has now been documented that some Palmer amaranth populations are also resistant to protoporphyrinogen oxidase inhibitor (PPO) herbicides.
Identifying Characteristics
Palmer amaranth is often confused with other amaranth species including redroot pigweed, spiny amaranth, smooth (also called green) amaranth, and slender amaranth. Of these five, Palmer amaranth is the only species with male and female plants. Palmer amaranth can be distinguished by the lack of a spine at the base of the petiole, absence of hairs on stem and leaf surfaces, and a petiole that is as long as or longer than its leaf (Weakley 2015). An easy way to determine the petiole length is to pull a leaf and petiole off the plant and bend the petiole back over the leaf blade to compare the petiole and leaf blade lengths. Long petioles are most evident on the older leaves of the plant present on the lower part of the stem. Slender amaranth and Palmer amaranth are particularly difficult to distinguish. Slender amaranth rarely grows taller than 3 feet and has a brown seed head; Palmer amaranth can grow taller than 7 feet and has a green seed head. Terminals on female Palmer amaranth have a main terminal seed head that can be as long as 3 feet. The seed heads also have stiff, sharp bracts that make the seed heads prickly to the touch.
Reproduction and Growth Habit of Palmer Amaranth
Palmer amaranth is an annual weed (Franssen et al. 2001) originating from northern Mexico and the southwestern United States (Ward et al. 2013). It can tolerate high temperatures better than many crops, with highest growth rates occurring between 97ºF and 115ºF (Ehleringer 1983). Palmer amaranth grows rapidly, at a rate of 2.5 inches a day (Smith et al. 2020). Its fast growth and establishment allow it to compete with the production crop for water, nutrients, and light. Each female plant can produce between 200,000 and 600,000 seeds per plant, depending on time of emergence (Keeley et al. 1987). Palmer amaranth is highly opportunistic—with optimal temperature and moisture, 100% of viable seed will germinate on the first day of planting (Steckel et al. 2004). Palmer amaranth grows faster and accumulates more dry matter than other Amaranthus species, including redroot pigweed (Sellers et al. 2003).
Impacts of Palmer Amaranth on Sweetpotato Yield and Quality
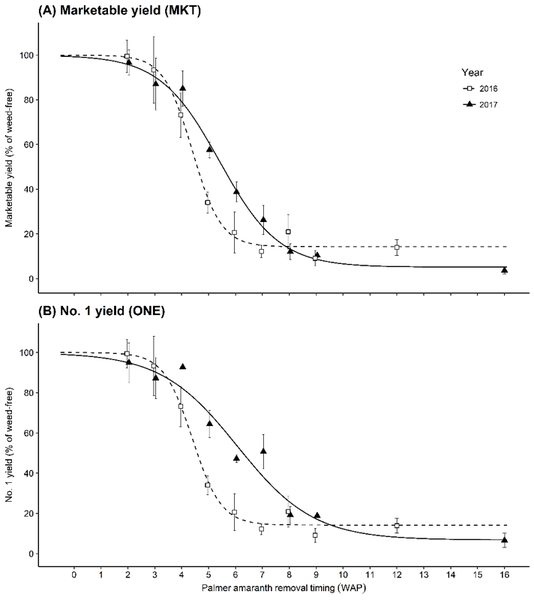
Previous research by Meyers et al. (2010) showed that Palmer amaranth can reduce sweetpotato marketable yield by as much as 81%. Even at low densities of a single Palmer amaranth plant per 3 feet of sweetpotato row, yield loss can be as high as 52%. Marketable (no.1 + jumbo) yield decreased 6% and 27%, respectively, when Palmer amaranth was allowed to germinate after sweetpotato transplanting and then removed at three and four weeks after transplanting (Figure 1). Full season Palmer amaranth interference resulted in 91% yield loss (Smith et al. 2020).
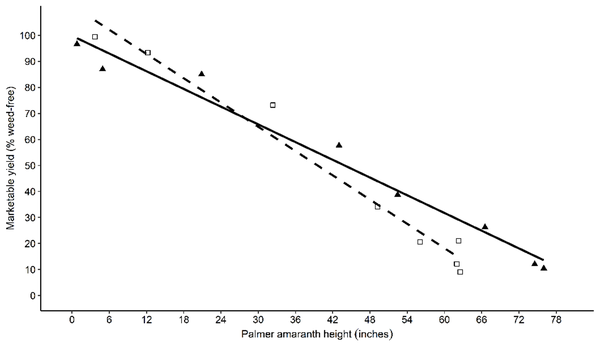
Palmer amaranth removed at two weeks after transplanting reduced no.1 yield by 5% and marketable yield by 4% (Figure 1). At three weeks after transplanting, Palmer amaranth can be about 5 inches tall; removal at this time can result in 13% reduction in marketable yield (Figure 2). Removal of Palmer amaranth beyond three weeks after transplanting can result in a 97% reduction in yield.
There is a strong correlation between Palmer amaranth height and sweetpotato marketable yield. For every inch of Palmer amaranth growth, marketable yield may be reduced by 1.25%. A 5% reduction in yield was observed by the time Palmer amaranth reached a height of 10.5 and 4.25 inches in 2016 and 2017, respectively (Figure 3).
Assuming a 5% acceptable marketable yield loss threshold, the critical time for Palmer amaranth removal in sweetpotato is two weeks after transplanting. Thus early-season scouting and early removal of Palmer amaranth in sweetpotato fields are critical. Any delay in removal can result in substantial yield reductions and fewer premium quality roots.
Managing Palmer Amaranth in Sweetpotato
Palmer amaranth emerges from March to October in North Carolina and is able to germinate, grow, and produce seed in about 30 days. Start with a clean seedbed. All plants should be controlled prior to seed production.
Herbicide options for Palmer amaranth control in sweetpotato are limited. Flumioxazin (Valor), fomesafen (Reflex), and S-metolachlor (Dual Magnum) are the only herbicides registered for use in sweetpotato that have activity on Palmer amaranth. Flumioxazin, fomesafen, and S-metolachlor have only preemergence activity on Palmer amaranth; once Palmer amaranth has emerged, S-metolachlor and flumioxazin will not control it. Flumioxazin and fomesafen should be applied to the preformed bed prior to transplanting sweetpotato slips. Fomesafen should not be applied alongside flumioxazin. Instead, growers should choose one or the other depending on the weeds expected and future crops. There are potential carryover concerns with fomesafen, so thoroughly read herbicide labels before use. Sweetpotato should be transplanted into weed-free beds. Therefore, it may be necessary to rework the beds prior to transplanting sweetpotato. It is much more difficult to control Palmer amaranth after sweetpotato has been planted and after Palmer amaranth has emerged. S-metolachlor should be applied to weed-free sweetpotato following a cultivation and/or a hand-weeding event 7 to 14 days after transplanting to reduce the risk of injury to the developing sweetpotato storage roots. If S-metolachlor is applied too soon after transplanting and heavy rains occur, injury, such as shortening and rounding of roots, can occur (Blankenship et al. 2024; Meyers et al. 2010; Meyers et al. 2013).
Other methods to control Palmer amaranth in sweetpotato include cultivation, hand removal, and mowing. Standard cultivation equipment can be effective at removing small (< 2 inches) Palmer amaranth in and between rows if cultivation is timely (K.M. Jennings, personal communication). Hand removal of Palmer amaranth is possible but very expensive ($200 to $400 per acre) compared to other weed control methods (Ted Burch, grower, personal communication in a telephone conversation Nov. 13, 2018). It can be difficult to remove Palmer amaranth by hand once it reaches about 18 inches tall because the stem has become woody and a deep taproot and network of fibrous roots have formed. It may be necessary to use a machete to cut the stem at the soil surface if the plant cannot be pulled by hand. Any removed plants must be taken away from the field and composted or burned. Plants left on the soil surface will reroot and continue to grow and produce viable seed. Broken stems as small as 1 inch can resprout, flower, and produce seed (Sosnoskie et al. 2014).
Light interception is one of the main mechanisms of Palmer amaranth competition in sweetpotato (Moore et al. 2021). If Palmer amaranth plants escape control and grow above the sweetpotato canopy, it can be beneficial to use a mower to control the portion of the weed that is above the crop canopy. Mow Palmer amaranth before seed maturity to avoid the seed being distributed onto the soil surface, where it may germinate the same season or in future years. After mowing, Palmer amaranth adopts a horizontal growth pattern. Though it will still compete with the crop for water and nutrients, it will no longer shade the crop and if mowed at the right time will produce fewer seeds.
Because Palmer amaranth has such small seeds, deep tillage can be done prior to bedding to bury seeds and prevent emergence. Deep tillage alone can reduce Palmer amaranth emergence the following growing season by 81% (DeVore 2012). After three years of burial at a depth of 4 inches, only 15% of Palmer amaranth seeds will remain viable (Sosnoskie et al. 2012). Deep tillage should not be done every year because seeds will be brought back to the soil surface.
References
Blankenship, C. D., K. M. Jennings, D. W. Monks, S. L. Meyers, D. L. Jordan, J. R. Schultheis, D. H. Suchoff, L. D. Moore, S. J. Ippolito. 2024. “Effect of S-metolachlor and flumioxazin herbicides on sweetpotato treated with and without activated charcoal applied through transplant water.” Weed Technology 38, e50. doi:10.1017/wet.2024.48 ↲
Burke, I. C., M. Schroeder, W. E. Thomas, and J. W. Wilcut. 2007. “Palmer amaranth interference and seed production in peanut.” Weed Technology 21(2): 367–371. ↲
Chandi, A., D. L. Jordan, A. C. York, S. R. Milla-Lewis, and J. D. Burton. 2012. “Interference of selected Palmer amaranth (Amaranthus palmeri) biotypes in soybean (Glycine max).” International Journal of Agronomy. ↲
DeVore, J. D., J. K. Norsworthy, and K. R. Brye. 2012. “Influence of deep tillage and a rye cover crop on glyphosate-resistant Palmer amaranth (Amaranthus palmeri) emergence in cotton.” Weed Technology 26 (4): 832–838. ↲
Ehleringer, J. 1983. “Ecophysiology of Amaranthus palmeri, a Sonoran Desert summer annual.” Oecologia 57:107–112. ↲
Franssen, A. S., D. Z. Skinner, K. Al-Khatib, M. J. Horak, and P. A. Kulakow. 2001. “Interspecific hybridization and gene flow of ALS resistance in Amaranthus species.” Weed Science 49 (5): 598–606. ↲
Meyers, S. L, K. M. Jennings, J. R. Schultheis, and D. W. Monks. 2010. “Interference of Palmer amaranth (Amaranthus palmeri) in sweetpotato.” Weed Science 58 (3):199–203. ↲
Meyers, S. L., K. M. Jennings, D. W. Monks, D. K. Miller, and M. W. Shankle. 2013. “Rate and application timing effects on tolerance of Covington sweetpotato to S-metolachlor.” Weed Technol 27 (4): 729–734. ↲
Meyers, S. L. and M. W. Shankle. 2016. “Postemergence yellow nutsedge management in sweetpotato.” Weed Technology 30 (1): 148–153. ↲
Moore L. D., K. M. Jennings, D. W. Monks, D. L. Jordan, R. G. Leon, M. D. Boyette. 2021. “Evaluating shade cloth to simulate Palmer amaranth (Amaranthus palmeri) competition in sweetpotato.” Weed Science 69 (4):478–484. doi:10.1017/wsc.2021.21 ↲
Morgan, G. D., P. A. Baumann, and J. M. Chandler. 2001. “Competitive impact of Palmer amaranth (Amaranthus palmeri) on cotton (Gossypium hirsutum) development and yield.” Weed Technology 15 (3):: 408–412. ↲
Seem, J. E., N. G. Creamer, and D. W. Monks. 2003. “Critical weed-free period for 'Beauregard' sweetpotato (Ipomoea batatas).” Weed Technology 17 (4): 686–695. ↲
Sellers, B. A, R. J. Smeda, W. G. Johnson, J. A. Kendig, and M. R. Ellersieck. 2003. “Comparative growth of six Amaranthus species in Missouri.” Weed Science 51 (3): 329–333. ↲
Smith, S. C., K. M. Jennings, D. W. Monks, S. Chaudhari, J. R .Schultheis, S. C. Reberg-Horton. 2020. Critical timing of Palmer amaranth (Amaranthus palmeri) removal in sweetpotato. Weed Technology 34:547–551 ↲
Sosnoskie, L. M., T. M. Webster, A. S. Culpepper, and J. Kichler. 2014. The Biology and Ecology of Palmer Amaranth: Implications for Control. Circular 1000. University of Georgia Extension. ↲
Sosnoskie, L. M., T. M. Webster, and A. S. Culpepper. 2012. “Glyphosate resistance does not affect Palmer amaranth (Amaranthus palmeri) seedbank longevity.” Weed Science 61 (2): 283–288. ↲
Steckel, L. E., C. L. Sprague, E. W. Stoller, and L. M. Wax. 2004. “Temperature effects on germination of nine Amaranthus species.” Weed Science 52 (2): 217–221. ↲
Ward, S. M., T. M. Webster, and L. E. Steckel. 2013. “Palmer amaranth (Amaranthus palmeri): A review.” Weed Technology 27 (1): 12–27. ↲
Weakley, A. S. 2015. Flora of the Southern and Mid-Atlantic States. University of North Carolina Herbarium, North Carolina Botanical Garden. Chapel Hill: University of North Carolina. ↲
Webster, T. M. 2010. “Weed survey-southern states: vegetable, fruit and nut crops subsection (annual weed survey).” Proceedings of the Southern Weed Science Society 63: 246–257. ↲
Publication date: Nov. 26, 2024
AG-857
There is an alternate English language version of this document here: Control del Amaranto Palmer en el Cultivo de Camote
Recommendations for the use of agricultural chemicals are included in this publication as a convenience to the reader. The use of brand names and any mention or listing of commercial products or services in this publication does not imply endorsement by NC State University or N.C. A&T State University nor discrimination against similar products or services not mentioned. Individuals who use agricultural chemicals are responsible for ensuring that the intended use complies with current regulations and conforms to the product label. Be sure to obtain current information about usage regulations and examine a current product label before applying any chemical. For assistance, contact your local N.C. Cooperative Extension county center.
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.