Tomatoes are attacked by a large number of insect pests from the time plants first emerge in the seedbed until harvest. Aphids, flea beetles, and leafminers threaten young transplant-bed tomatoes. In the field, flea beetles, aphids, leafminers, stink bugs, and tomato fruitworms cause minimal damage to the foliage; however, severe damage may result either from their feeding on the fruit or by their spreading of diseases.
Greenhouse tomatoes have many of the same pests as field tomatoes. Tiny pests such as aphids, whiteflies, leafminers, and twospotted spider mites, however, are more likely to infest greenhouse crops than beetles, grubs, or caterpillars. Occasionally, moths enter through holes in screens or fans and lay eggs in greenhouses. Even in screened greenhouses, tomato fruitworms and cabbage loopers may be brought into the greenhouses on plants.
A. Pests that feed on the upper plant
- Pests that mine leaves or bore into fruits or buds
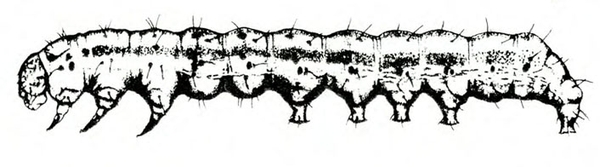
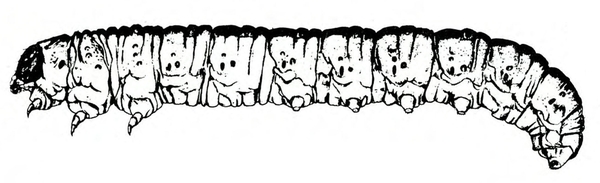
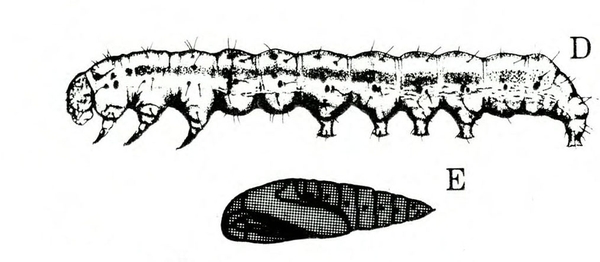
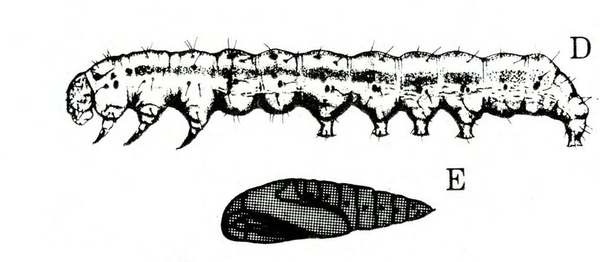
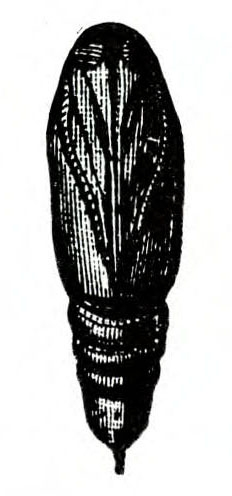
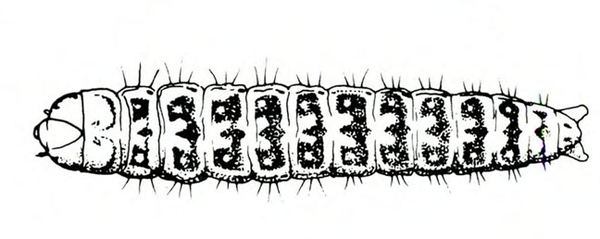
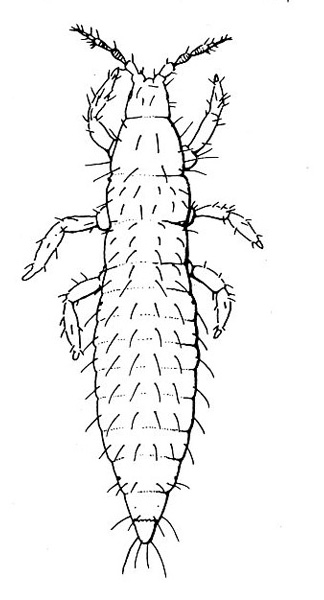
- Tomato fruitworm (also known as corn earworm)—Early instars are cream colored or yellowish green with few markings. Later instars are green, reddish, or brown, with pale stripes and scattered black spots, moderately hairy, and up to 1 3/4 inches long, with three pairs of legs and five pairs of prolegs (Figure 1). These larvae chew holes in fruits and buds.
- Tobacco budworm—This caterpillar looks similar to the tomato fruitworm, except mature worms are somewhat smaller and slightly more slender than tomato fruitworms (Figure 2); in addition, the microscopic spines on the skin of tobacco budworms are more slender, longer, and occur closer to the setae (hairs).
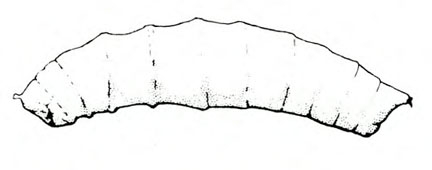
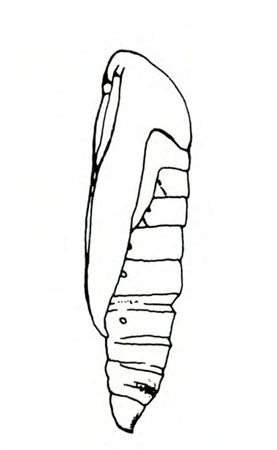
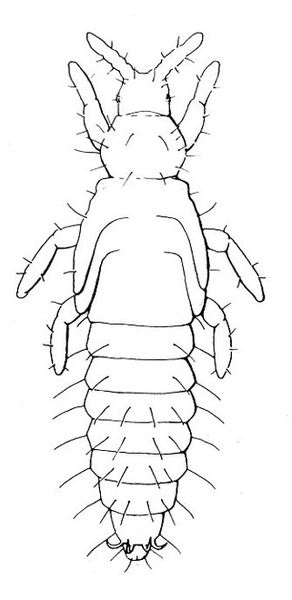
- Tomato pinworm—Tiny, yellowish-gray larvae make blotch mines in leaves. Older yellow, green, or gray purple-spotted larvae grow up to 5/16 inch long (Figure 3); they fold leaves and web them together or bore into stems, buds, and fruit. Fruits will have pinholes and discolored blotches.
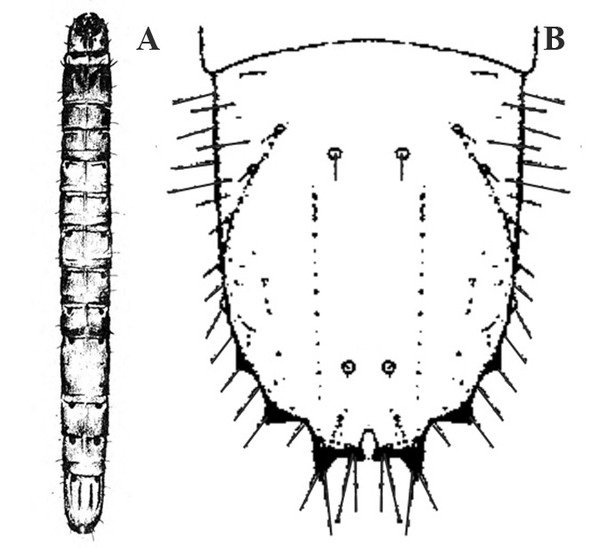
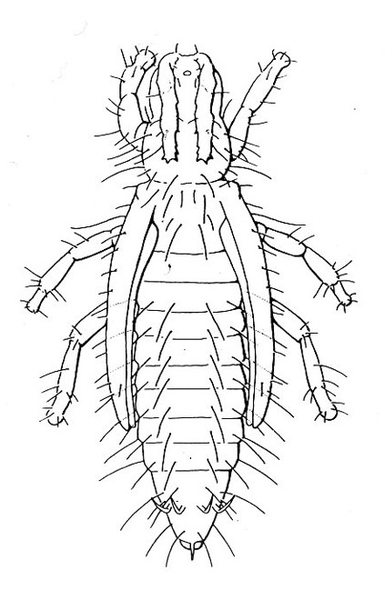
- Vegetable leafminer—The larva is a colorless to bright-yellow maggot, up to 1/8 inch long, with a tapered head (Figure 4). Larvae make serpentine mines in leaves, with each mine slightly enlarged at one end.
- Pests that chew holes in leaves
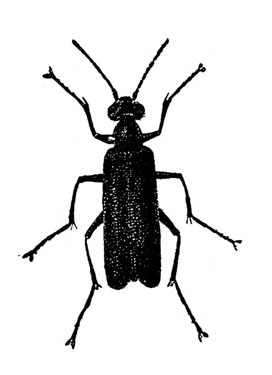
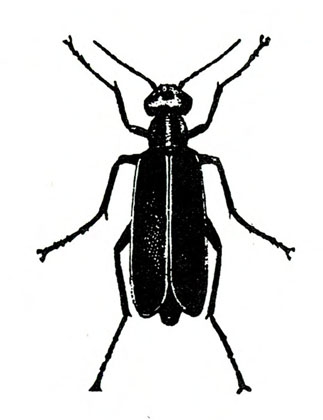
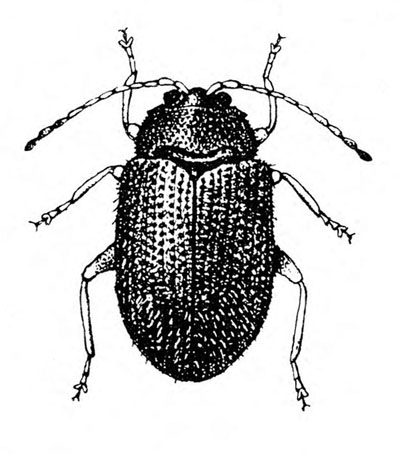
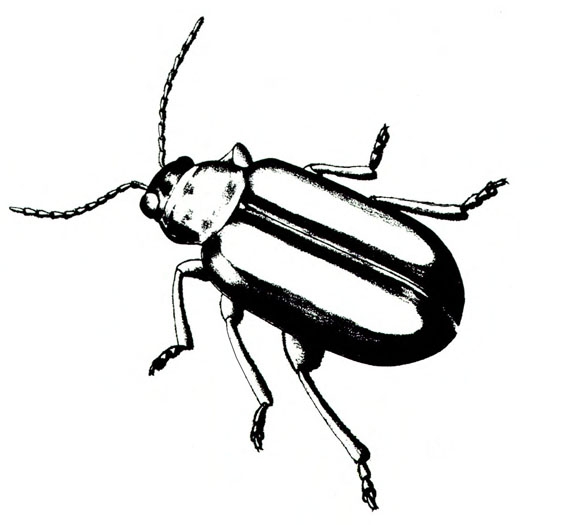
- Blister beetles—Several species of slender, elongate beetles with prominent heads grow up to 3/4 inch long. Bodies are variously colored but usually black (Figure 5A), black with yellow margins (Figure 5B), or black-and-yellow striped (Figure 5C). They leave stringy, black excrement on heavily infested plants. Foliage is raggedly chewed, and plants are sometimes stunted. (For more information about blister beetles, see “Pests of Potato.”)
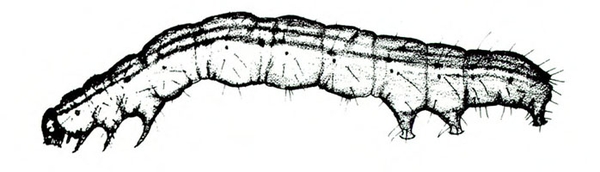
- Cabbage looper—This green caterpillar has longitudinal white stripes. Its body is up to 1 3/16 inches long, tapering toward the head (Figure 6). It has three pairs of legs near the head and three pairs of fleshy prolegs. Young larvae hang out on the undersides of leaves, consuming tender leaf tissue while leaving most veins intact. (For more information about cabbage loopers, see “Pests of Crucifers.”)
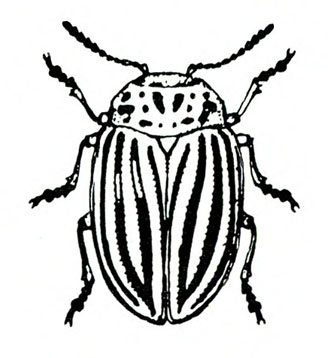
- Colorado potato beetle—These yellowish-brown, oval, convex beetles (Figure 7) are up to 9/16 inch long with five black longitudinal stripes on each wing cover and several black spots on the pronotum (area behind the head). They feed on leaves and terminal growth. (For more information about Colorado potato beetles, see “Pests of Potato.”)
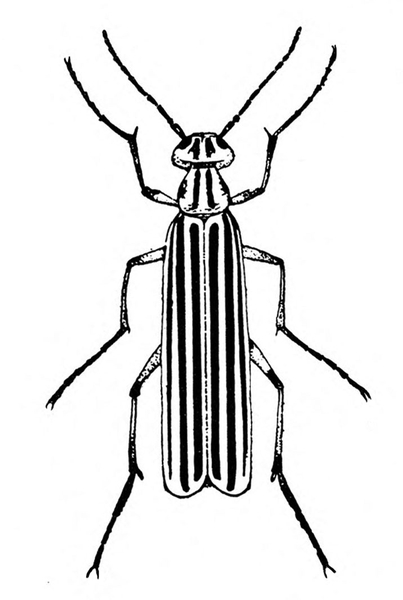
- Flea beetles—These various species of tiny, dark-colored beetles, 1/8 to 3/16 inch long, have solid-brown to black bodies (Figure 8A), brown bodies with dark spots (Figure 8B), or black bodies with a pale-yellow stripe on each wing cover (Figure 8C). Their feeding leaves tiny, round holes in foliage. (For more information about flea beetles, see “Pests of Potato.”)
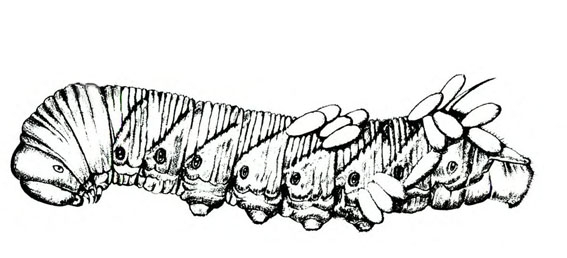
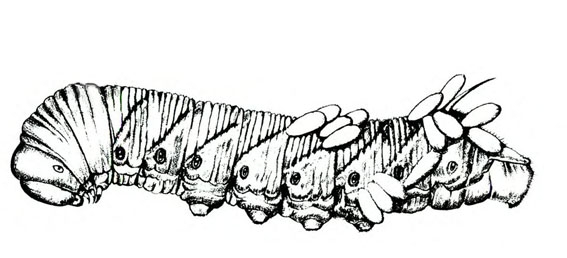
- Hornworms—These green to reddish-brown caterpillars are up to 3 1/2 inches long with a red or black anal horn. The body has seven diagonal marks (tobacco hornworm, Figure 9B) or eight V-shaped marks on each side (tomato hornworm, Figure 9A) and round, black spiracles along the side of the body. Hornworms strip leaves from vines and infrequently feed on fruit, leaving large, open, superficial scars.
- Sap-sucking pests that cause leaf discoloration, leaf or fruit deformation, or defoliation
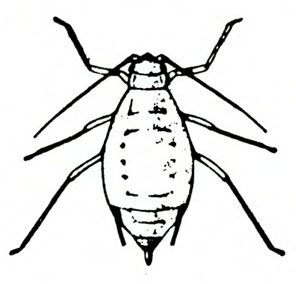
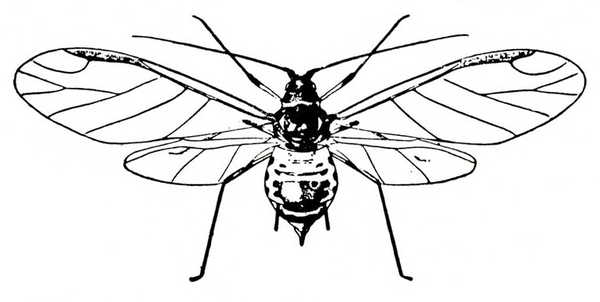
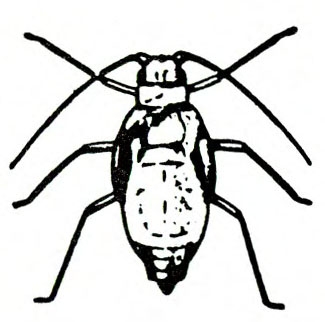
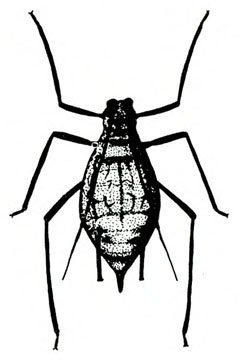
- Aphids—Soft-bodied, pear-shaped insects with a pair of dark cornicles and a cauda protruding from the abdomen, aphids may be winged or wingless (most common). They feed in colonies, causing discoloration or mottling of the foliage and excreting honeydew on which sooty mold grows.
- Green peach aphid—The pale-yellow to green wingless adult is almost 1/8 inch long (Figure 10A). The winged adult has a dark, dorsal blotch on a yellowish-green body (Figure 10B). The nymph has three dark lines on its abdomen (Figure 10C). (For more information about green peach aphids, see “Pests of Lettuce.”)
- Potato aphid—Adults and nymphs are solid pink, mottled green-and-pink, or light green with a dark stripe. Adults are a little more than 1/8 inch long with long, slender cornicles about twice as long as the cauda (Figure 11). (For more information about potato aphids, see “Pests of Lettuce.”)
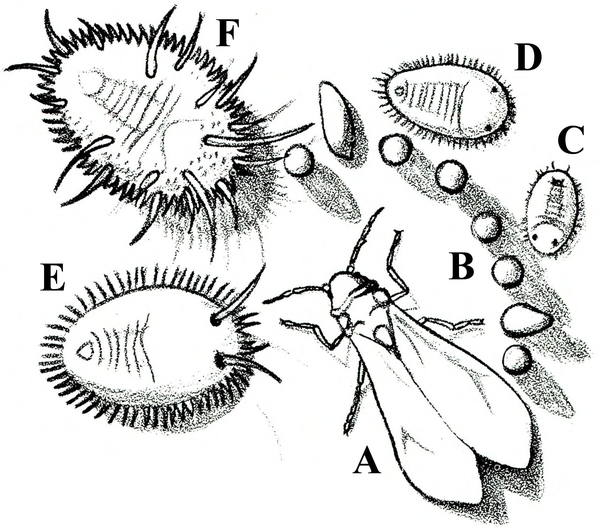
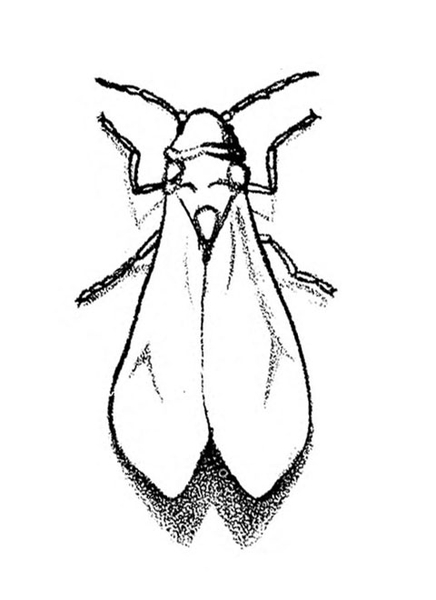
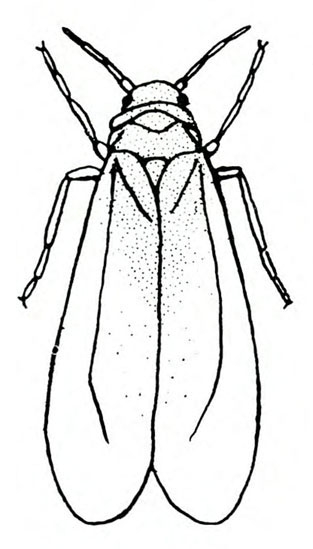
- Greenhouse whitefly—This white, mothlike insect is about 1/16 inch long. It is found in conjunction with tiny, yellow crawlers (first instars, the only immature stage that is mobile); green, oval, flattened, immobile nymphs; or pupae (Figure 12A–F). Their feeding activity causes leaves to yellow and drop from the plant. Some plants may appear stunted and cease blooming, with black, sooty mold often present on leaves.
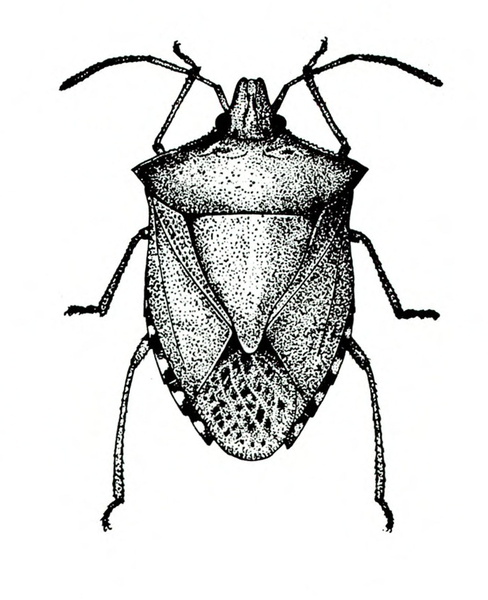
- Stink bug—A green or brown shield-shaped insect, the adult is up to 3/4 inch long (Figure 13). The nymph is green with orange and black markings. Stink bugs pierce buds and fruit, causing buds to drop and fruit to be deformed. (For more information about stink bugs, see “Pests of Okra.”)
- Silverleaf whitefly—The adult is slightly smaller than the greenhouse whitefly (females are almost 1/16 inch long, with males smaller) and slightly more yellow in color. It holds its wings rooflike at about a 45-degree angle. Nymphs appear glassy to opaque yellowish and have a flattened, scalelike body with the margin near the leaf surface (Figure 14A–D). The pupa is slightly rounded, its margins slope inward, and it lacks noticeable setae. Infested plants are stunted and nonreproductive with black, sooty mold present.
- Twospotted spider mite—Tiny (almost microscopic) and pale to dark green, twospotted spider mites have two or four dark-colored spots. Adults and nymphs have eight legs (Figure 15). Larvae have six legs. Females are oval and about 1/64 inch long. Males are tapered toward the rear. Infested foliage becomes silvery because of pale-yellow stipples. Leaves eventually become pale and die. Mites spin silken webs on the undersides of leaves as they feed. (For more information about twospotted spider mites, see “Pests of Beans and Peas.”)
- Western flower thrips—The adult, almost 1/16 inch long, varies from pale yellow to dark brown and has a rounded, narrow abdomen (Figure 16). Larvae are distinctly yellow. Infested plants are distorted and have a silvery appearance. Western flower thrips are an important vector of tomato spotted wilt virus.
- Aphids—Soft-bodied, pear-shaped insects with a pair of dark cornicles and a cauda protruding from the abdomen, aphids may be winged or wingless (most common). They feed in colonies, causing discoloration or mottling of the foliage and excreting honeydew on which sooty mold grows.
B. Pests that feed on roots or lower stems
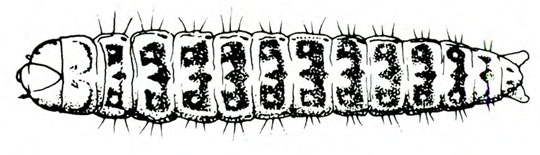
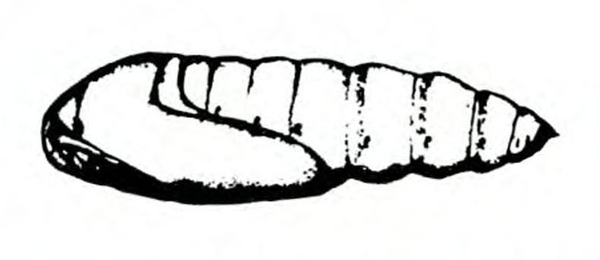
- Cutworm—This fat, basically gray, brown, or black caterpillar is 1 5/8 to 2 inches long when fully grown, with three pairs of legs near the head and five pairs of fleshy prolegs (Figure 17). They are active at night. The young caterpillar climbs on leaves, and the older caterpillar severs seedling stems near the ground. They hide during the day in soil burrows at the bases of plants. (For more information about cutworms, see “Pests of Asparagus.”)
- Southern potato wireworm—The slender, wirelike, cylindrical larva has three pairs of short legs near the head, a pair of fleshy anal prolegs, and a white, cream, or yellow-gray body with a red-orange head capsule. It is about 11/16 inch long when fully grown, with a closed notch in the last abdominal segment (Figure 18A–B). Their feeding causes ragged, irregular holes in roots. (For more information about southern potato wireworms, see “Pests of Potato.”)
Cutworms
Black cutworm, Agrotis ipsilon (Hufnagel), Noctuidae, LEPIDOPTERA
Granulate cutworm, Feltia subterranea (Fabricius), Noctuidae, LEPIDOPTERA
Variegated cutworm, Peridroma saucia (Hübner), Noctuidae, LEPIDOPTERA
Description
Adult—Black cutworm moths have dark-brown forewings, light-colored hind wings, and a wingspan of 1 1/2 to 2 inches (Figure 19A–B). Granulate cutworm moths have yellowish-brown forewings and a wingspan of 1 1/2 to 1 3/4 inches (Figure 19E). Variegated cutworm moths may be yellowish or brownish with a wingspan of 1 1/2 to 2 inches (Figure 19H).
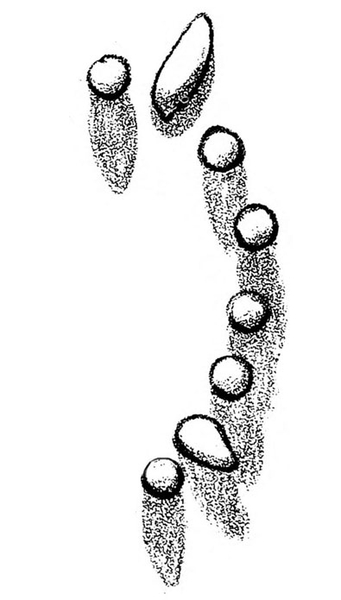
Egg—Cutworm eggs are white and usually laid singly or in small clusters (Figure 19A–B). Variegated cutworm eggs are generally laid in elongate patches (Figure 19I–J).
Larva—Black cutworm larvae are dark-greasy-gray to black with a light-colored line down the center of the back. They are 1 1/2 to 1 3/4 inches long when mature, and the skin is covered with smooth, black granules (Figure 19C). Granulate cutworm larvae are dusty brown with a more roughly granulate skin and 1 to 1 1/2 inches in length (Figure 19F). Variegated cutworm larvae have a distinct, pale-yellow dot on the middorsal line of each segment. About 2 inches long when mature, they are pale dirty-brown in color (Figure 19K).
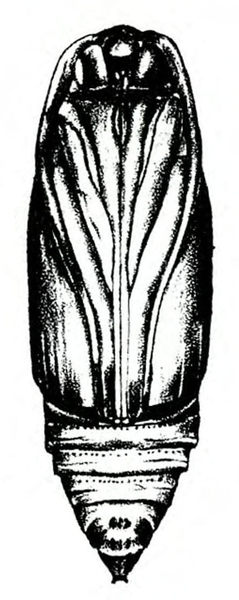
Pupa—Cutworm pupae are about 3/4 inch in length and dark brown or mahogany in color (Figure 19D, Figure 19G, and Figure 19L).
Biology
Distribution—Cutworms are cosmopolitan in their distribution and are common in Canada and the United States. The black cutworm is more abundant in the northern portions of its range, while the granulate cutworm is more abundant southward. In North Carolina, cutworms are generally more of a problem in the coastal plain.
Host Plants—Besides tomato, cutworms attack many field crops, grasses, and vegetable crops such as asparagus, bean, crucifers, cucurbits, corn, cowpea, lettuce, onion, pea, pepper, potato, spinach, and sweetpotato.
Damage—Several species of cutworms may injure vegetable seedlings and newly set plants in the field. Larvae hide under dirt clods and in cracks in the soil by day and emerge at night to cut off young plants near the ground and feed on the foliage. The black cutworm is one of the most destructive cutworms. One larva often severs numerous plants in a row during a single night. Small populations can cause considerable injury, resulting in the need to replant. The granulate cutworm and the mature variegated cutworm also sever plants. Early instars of the variegated cutworm will climb plants to feed on leaves.
Life History—Cutworms pass the winter as larvae or pupae. In early spring, larvae that have overwintered resume activity and feed until they are mature, then pupate in the soil. Moths emerge from pupae two to four weeks later. Soon afterward, females begin depositing eggs at night in clusters on the undersides of leaves. Each female lays hundreds of eggs. Under favorable (warm) conditions, eggs hatch in three to five days.
Young larvae may feed on leaves, but larger larvae are most commonly found in the soil around the bases of plants. After feeding for three to four weeks and developing through five to eight instars, larvae pupate in the soil. A new generation of moths soon emerges. Most cutworms complete three or four generations per year in North Carolina.
Control
Because extensive damage may occur in a short period, plant beds and newly set plants should be inspected frequently. A bait may be used in infested plant beds or on newly set plants in the field. For control recommendations, consult the North Carolina Agricultural Chemicals Manual.
Greenhouse Whitefly
Trialeurodes vaporariorum (Westwood), Aleyrodidae, HEMIPTERA
Description
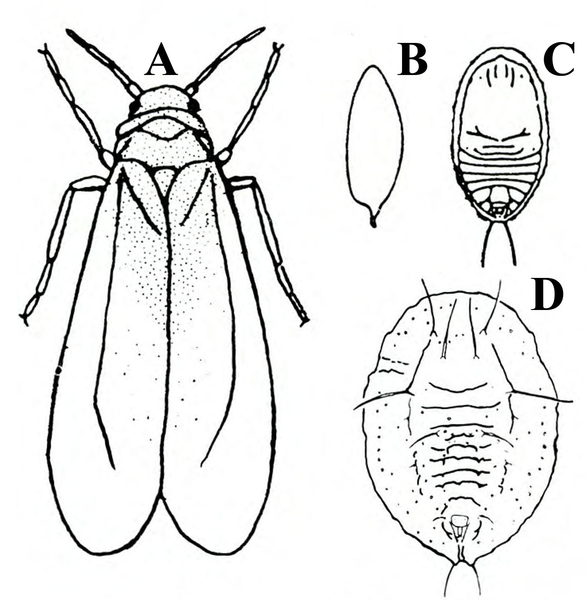
Adult—About 1/16 inch long, the adult is a white insect that resembles a tiny moth (Figure 20A).
Egg—The oblong eggs, which are pale green to purple, are inserted into the lower leaf surface, often in a circle or crescent pattern (Figure 20B). Each egg is about 1⁄128 inch long.
Nymph—The tiny, first-instar nymph (or crawler) is yellow with red eyes and functional legs and antennae (Figure 20C). Second (Figure 20D) and third (Figure 20E) instar nymphs are flattened, immobile, and scalelike.
Pupa—About 1/32 inch long, the oval, flattened pupa is pale green (healthy) or black (parasitized), with long hairs on its back and a fringe of short hairs around its body margin (Figure 20F). After the adult has emerged, a white, almost-transparent pupal skin is left behind.
Biology
Distribution—Greenhouse whiteflies are worldwide pests of greenhouse-grown ornamentals and vegetables. First discovered in England in 1856, they were found in the northeastern United States in 1870. Tropical Central America or South America are suggested origins of the greenhouse whitefly.
Host Plants—Greenhouse whiteflies infest a wide variety of ornamental and vegetable crops, and they can survive outdoors during the growing season, particularly in sheltered locations. Some of the more important hosts besides tomato include bean, cucumber, eggplant, grape, lettuce, melon, potato, squash, strawberry, and tobacco. Even trees, such as avocado, may be infested.
Damage—Nymphs and adults extract plant sap through their needlelike mouthparts, with the adults preferring to feed on tender new growth. As a result, leaves turn yellow and drop from infested plants. In addition to having an unthrifty appearance, plants may be stunted and cease flowering. Sooty mold often grows on leaves covered with honeydew secreted by whiteflies and interferes further with the growth of plants.
Life History—Greenhouse whiteflies reproduce relatively slowly (one generation every 30 to 45 days), but each female lays about 250 eggs and lives as long as two months. Adults usually are found on the lower surface of new leaves (Figure 21). There, they insert their eggs, which hatch five to seven days later. New crawlers move about the plant for a day or two, often from leaf to leaf, before inserting their mouthparts to feed. Once this occurs, they probably do not move again until mature. The crawlers molt into later nymphal instars and then into pupae. Finally, a new generation of whitish-yellow adults emerges. They soon are covered with a fine, white, waxy powder.
Control
Parasitic wasps, Encarsia formosa, sometimes help keep greenhouse whitefly populations under control. At temperatures below 75°F, the reproduction of these wasps is inhibited. As a result, whitefly infestations are favored by lower greenhouse temperatures.
Control of whiteflies is difficult because the eggs and immature forms are resistant to many aerosol and insecticide sprays. It is necessary to regularly apply pesticides to control emerging adults until the last of an entire generation of immature whiteflies has emerged. For specific chemical control recommendations, consult the North Carolina Agricultural Chemicals Manual.
Hornworms
Tobacco hornworm, Manduca sexta (Linnaeus), Sphingidae, LEPIDOPTERA
Tomato hornworm, Manduca quinquemaculata (Haworth), Sphingidae, LEPIDOPTERA
Description
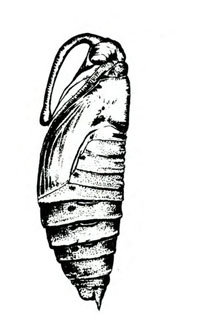
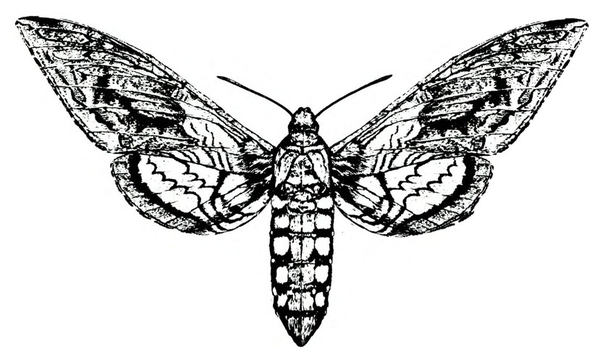
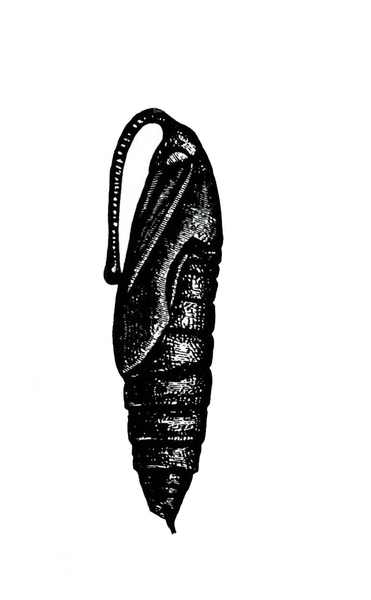
Adult—Hornworm moths have a wingspan of 4 to 5 inches. Tobacco hornworm moths are slate brown; tomato hornworm moths are ash gray. Tobacco hornworm moths have six orange spots on each side of the abdomen (Figure 22A); tomato hornworm moths have five similar, but less distinct, spots on each side (Figure 22E). The wavy lines on the hind wings of the tomato hornworm are more distinct and jagged than the lines on the hind wings of the tobacco hornworm moth.
Egg—Hornworm eggs are smooth, spherical, and almost 1/16 inch in diameter. Light green at first, they turn white before hatching.
Larva—Mature tobacco hornworm larvae usually have green bodies with fine, white pubescence and seven diagonal stripes on each side; the posterior horn is usually curved and red (Figure 22B). Tomato hornworm larvae have eight V-shaped markings on each side; the horn is straight and black (Figure 22F). The larvae of both species are about 3 to 3 1/3 inches long when fully grown.
Pupa—Pupae are brown, hard, spindle-shaped, and about 2 inches long. They have a curved, projecting tongue case shaped like a pitcher handle. The tongue case of the tomato hornworm (Figure 22G) is longer and more curved than the tongue case of the tobacco hornworm (Figure 22D).
Biology
Distribution—The tobacco hornworm ranges from southern Canada to Argentina. The range of the tomato hornworm extends only from southern Canada through the southern United States.
Host Plants—Hornworms feed primarily on solanaceous plants. Besides tomato, these hosts include tobacco, eggplant, pepper, and some weedy plants. They prefer tobacco and tomato plants for oviposition (egg-laying).
Damage—Hornworms strip leaves from tomato vines. If a heavy infestation develops, caterpillars also feed on developing fruit. Rather than bore into fruit, they feed superficially, leaving large, open scars. Fruit damage is much less common than defoliation. Hornworm damage usually begins in midsummer and continues throughout the remainder of the growing season.
Life History—Hornworms overwinter in the soil as pupae. Moths of this overwintering generation begin to emerge in early June and may continue to emerge as late as August. Nocturnal in habit, hornworm moths frequently can be seen hovering over plants at dusk. At night, they deposit eggs on the undersides of leaves. Each moth deposits one to five eggs per plant visit and may lay as many as 2,000 eggs. Hornworms emerge from the eggs about four days later, depending upon temperature. After feeding for three weeks, hornworms burrow into the soil and spend four days as prepupae. In summer, the pupal period lasts three weeks, after which a new generation of moths emerges. Heavy egg deposition is common in August and early September. At least two generations occur each year in North Carolina.
Control
In small gardens, hornworms can be controlled by picking the larvae off plants. In most seasons, hornworms are kept below economically damaging levels by a wasp parasite, Cotesia congregata. Parasitized hornworms are easily recognized by the small, white, oblong cocoons attached to their backs (Figure 22B and Figure 22C). Leave these worms in the garden so the emerging wasps can parasitize other hornworms. For chemical recommendations, consult the North Carolina Agricultural Chemicals Manual.
Silverleaf Whitefly
Silverleaf whitefly, Bemisia argentifolii Bellows and Perring, Aleyrodidae, HOMOPTERA
Description
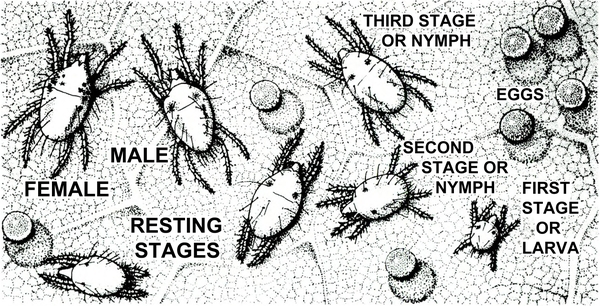
Adult—The silverleaf whitefly (Figure 23A) is slightly smaller and slightly yellower than other whiteflies. The female is almost 1/16 inch long, and the male is somewhat smaller (Figure 24). The wings are held rooflike at about a 45-degree angle, whereas other whiteflies usually hold their wings nearly flat over the body. Hence, the silverleaf whitefly appears more slender than other common whiteflies.
Egg—The eggs are whitish to light beige with the apex tending to be slightly darker. The eggs are inserted on end into the undersides of new leaves (Figure 23B).
Nymph—The nymphal stage appears glassy to opaque yellowish and may or may not have dorsal spines, depending on leaf characteristics. The body is flattened and scalelike, with the margins relatively near the leaf surface (Figure 23C and Figure 23D).
Pupa—The pupa or fourth nymphal instar is somewhat darker beige-yellow and opaque. Pupae are plumper than previous nymphal stages (Figure 24). Some of the spiracular furrows have a small amount of white wax deposits. The caudal setae are prominent, and the caudal end is somewhat acute. Dorsal spines are present when the host leaf is hairy and absent when the host leaf is smooth. The outer edges of the body are rounded.
Biology
Distribution—The silverleaf whitefly occurs around the world in tropical and subtropical areas and in greenhouses in temperate areas.
Host Plants—The number of host plants is extensive. The plant families most often reported as hosts are Amaranthaceae, Compositae, Convolvulaceae, Cruciferae, Cucurbitaceae, Euphorbiaceae, Labiatae, Leguminosae, Malvaceae, Moraceae, Rosaceae, Solanaceae, and Verbenaceae. In addition to tomato, the most frequently reported hosts in the southeastern United States are poinsettia and gerbera daisy.
Damage—Adults and nymphs cause direct damage by removing sap. Chlorotic spots sometimes appear at the feeding sites on leaves, and heavy infestations cause leaf wilting. The excretion of honeydew and the subsequent development of sooty mold fungi also may reduce photosynthesis and other physiological functions of the plant. Even though the silverleaf whitefly is considered an economic pest, little information is known about the damage it causes or the economic thresholds in tomatoes.
Life History—Developmental times from egg deposition to adult emergence vary from 16 to 38 days and appear to be primarily influenced by temperature, humidity, and the host plant. The number of eggs laid by each female over her lifetime varies considerably, but is usually about 90. Reports from Israel indicate that repeated applications of insecticides have produced a resistant, highly fecund strain of silverleaf whitefly, with the females capable of laying 300 eggs each. Apparently at temperatures above 97°F, eggs fail to hatch. "Crawlers" hatch from the eggs and crawl around until they insert threadlike mouthparts into the underside of the leaf to feed. They tuck their legs and antennae underneath their body and settle down close to the leaf surface.
Crawlers molt into scalelike nymphs that also suck out sap. Nymphs molt a second and third time. The fourth stage eventually becomes a nonfeeding pupa. Adult whiteflies develop within the pupa. Adults emerge from the pupa through a T-shaped slit about a month from the time the egg was laid. Females live about two weeks.
Control
Control of silverleaf whiteflies is difficult because the eggs and older immature forms are resistant to many aerosol and insecticide sprays; in addition, the adults are extremely resistant to dry pesticide residue. For adequate control, the pesticide mixture must be directed to the lower leaf surface, where all stages of the whiteflies naturally occur. Pesticides must be regularly applied to control crawlers and second stage nymphs until the last of an entire generation of immature whiteflies has hatched. Some of the pyrethroid pesticides, however, are somewhat more effective and need not be applied as often. Neem seed extract is not as acutely toxic as some of the synthetic pesticides, but it has the advantage of being toxic to young nymphs, inhibiting growth and development of older nymphs and reducing oviposition by adults. For specific chemical control recommendations, consult the North Carolina Agricultural Chemicals Manual.
Tomato Fruitworm and Tobacco Budworm
Tomato fruitworm (also known as corn earworm), Helicoverpa zea (Boddie), Noctuidae, LEPIDOPTERA
Tobacco budworm, Heliothis virescens (Fabricius), Noctuidae, LEPIDOPTERA
Description
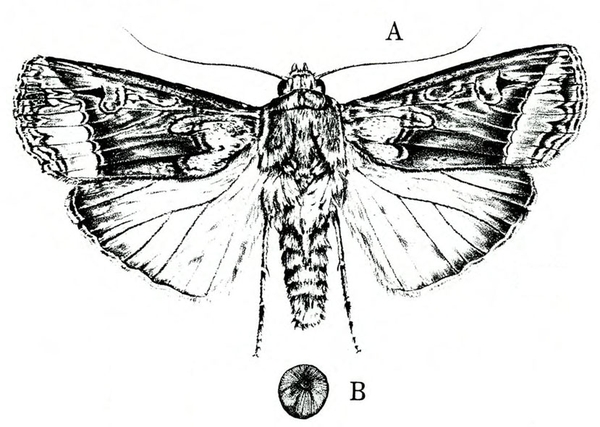
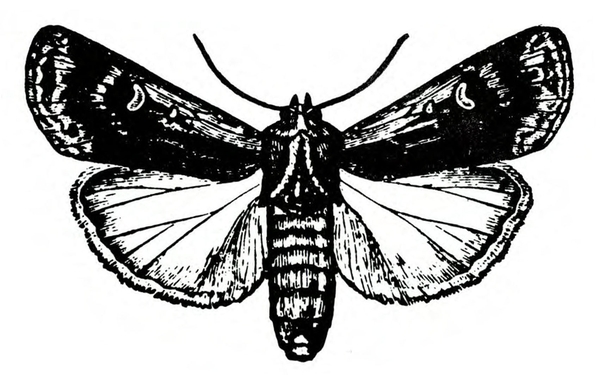
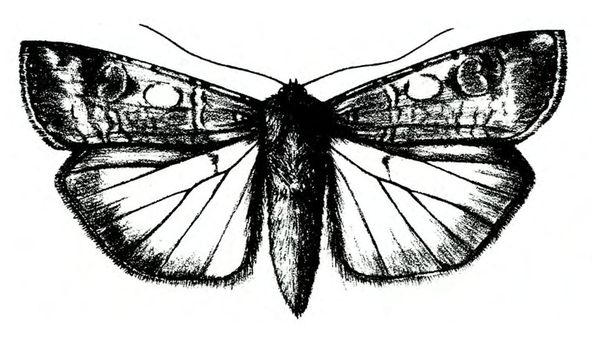
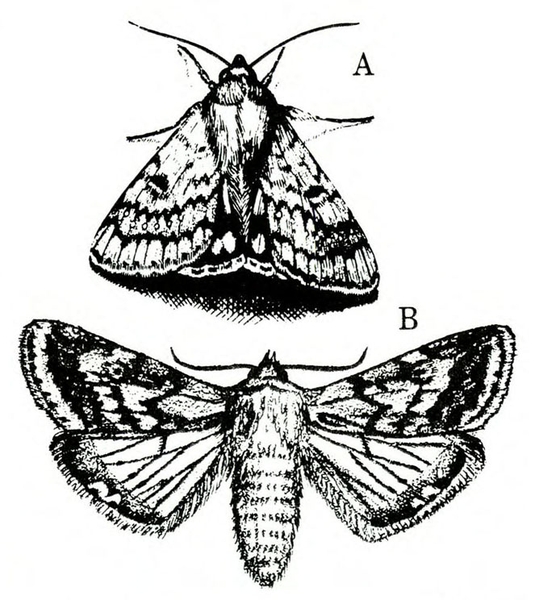
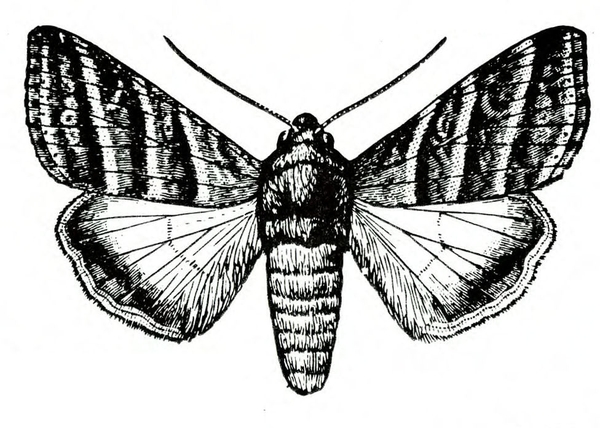
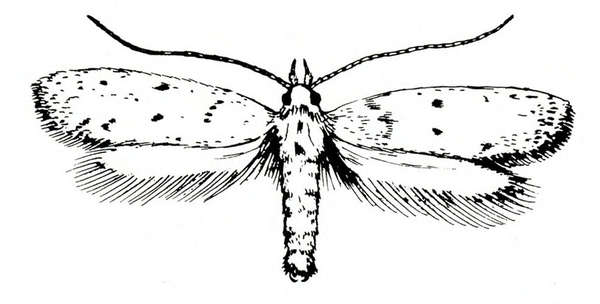
Adult—The tomato fruitworm moth is usually light yellowish-olive with a single dark spot near the center of each forewing. It has a wingspan of 1 to 1 1/2 inches (Figure 25A–B and Figure 25C). The eyes are usually light green. Tobacco budworm moths are light olive to brownish olive with a wingspan of 1 1/4 to 1 1/2 inches (Figure 25F). Each forewing bears three slanted dark-olive or brown bands. Hind wings are white with white borders.
Egg—Eggs of both species are similar in appearance. They are almost spherical with a flattened base, about 1/32 inch in diameter, and white or cream colored. They develop a reddish-brown band just prior to hatching.
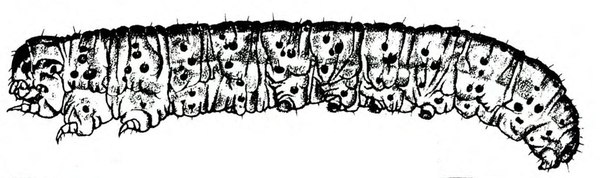
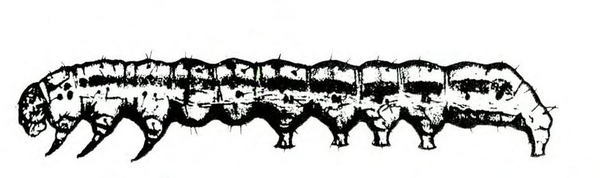
Larva—The tomato fruitworm (Figure 25D–E) and tobacco budworm (Figure 25G) are similar in appearance. Newly emerged larvae are yellowish white with a brown head. Color varies from greenish yellow and reddish brown to black, with paler stripes running lengthwise on the body. The skin of the tobacco hornworm has microscopic spines that are longer and closer to the setae than those of the tomato fruitworm.
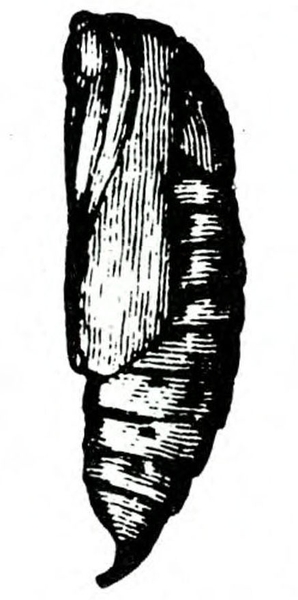
Pupa—Pupae of both species are typical for this family of “owlet” moths (Figure 25D–E and Figure 25H). Shiny and reddish brown at first, they become dark brown before the adult emerges.
Biology
Distribution—Tomato fruitworm occurs throughout the Western Hemisphere, extending as far north as Canada and as far south as Argentina. The tobacco budworm has a similar distribution but is more abundant in warmer regions, whereas the tomato fruitworm is more abundant in cooler regions. In North Carolina, fruitworms occur throughout the state but are generally more severe in the southern coastal plain.
Host Plants—The tomato fruitworm feeds on at least 16 cultivated plants, with corn the most important host. The tobacco budworm does not infest corn. Both species are common on cotton and soybeans. Tomato, cotton, soybean, and tobacco are the only cultivated crop hosts of the tobacco budworm in North Carolina. Wild hosts include deergrass and toadflax.
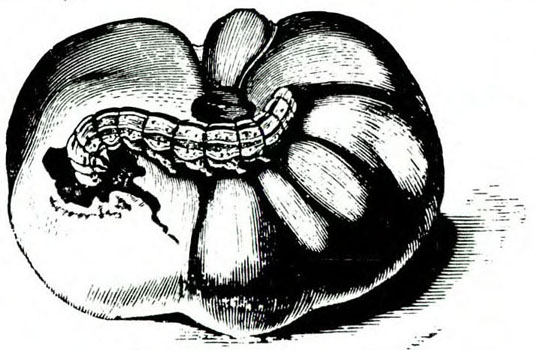
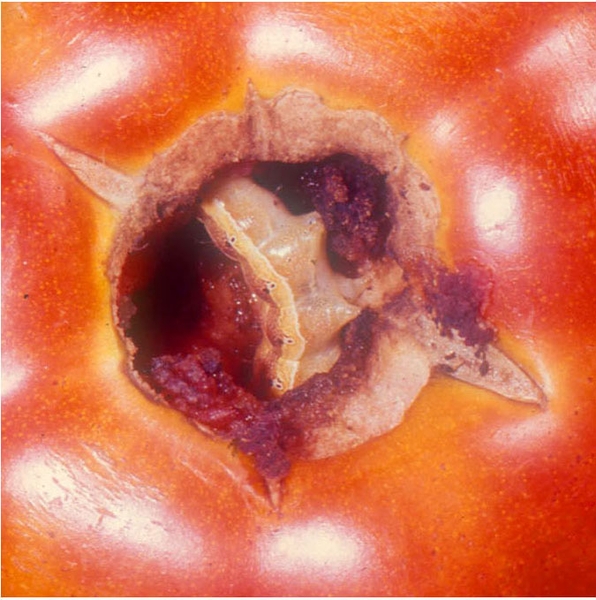
Damage—Fruitworms, primarily the tomato fruitworm, feed on tomato leaves and fruit (Figure 25I and Figure 25J). Distorted leaves often result from the worms' feeding on the tips of the leaves in the developing bud. Both the tomato fruitworm and tobacco budworm may also bore into stalks or midribs.
As tomato plants flower and produce fruit, tomato fruitworm larvae move to these plant parts. Entrance holes of small larvae in fruit may be barely detectable. As infested tomatoes increase in size, small, stringlike scars may become apparent where the tiny larvae entered. Large tomato fruitworms may move from fruit to fruit as they feed, leaving large, gaping holes on the surface.
Life History—Fruitworms overwinter as pupae in the soil. Tomato fruitworm adults emerge from early May to early June. Females generally emerge earlier than males. Tobacco budworm adults emerge from late April to mid-May. They deposit eggs on the leaves or buds of tomato plants. After hatching, larvae may first feed on leaves and then move to buds or fruit. Tomato fruitworm larvae have five to six instars, with the development period varying from 21 to 25 days. Tobacco budworm development is similar. Pupation occurs in the soil. Tomato fruitworm pupae enter diapause (dormancy) in August in North Carolina, and tobacco budworms begin diapause in September. Both species have four generations per year in North Carolina.
Control
The wasp parasite Campoletis sonorensis kills small tobacco budworms; another wasp parasite, Cardiochiles nigriceps, kills budworms in the prepupal stage. Predators include several paper wasps in the Polistes genus. Several diseases, including the microsporidian Nosema heliothidis Lutz and Splendor, also reduce budworm populations.
Tomato fruitworm damage is minimal when the fruits are harvested before July 20. The first chemical treatment should be applied when tomatoes in the first cluster are about 1/2 inch in diameter. Apply follow-up treatments every seven to ten days as necessary. In the coastal plain, a five- to seven-day schedule is needed after July 20. For specific chemical control recommendations, consult the North Carolina Agricultural Chemicals Manual.
Cultivating vegetable fields after harvest kills numerous pupae in the soil and exposes many to birds and other predators.
Tomato Pinworm
Tomato pinworm, Keiferia lycopersicella (Walsingham), Gelechiidae, LEPIDOPTERA
Description
Adult—This small gray moth has a reddish-brown mottled head and thorax. Its body is about 1/4 inch long, and its wingspan is 3/8 to 1/2 inch (Figure 26A).
Egg—The tiny, oval egg is light yellow when newly deposited but turns pale orange before hatching (Figure 26B).
Larva—Tiny, newly hatched larvae are yellowish gray. The mature fourth instar may be yellow, green, or ash-gray and is covered with dark-purple spots; it is about 1/4 inch long (Figure 26C).
Pupa—A little over 1/4 inch long, the pupa gradually changes from green to brown (Figure 26D). It is found in the soil enclosed in a pupal cell made of loosely woven silk and covered with soil particles.
Biology
Distribution—This pest lives year-round in the warm agricultural areas of Mexico, California, Florida, and Texas. Farther north, pinworms are primarily a greenhouse pest, although they may infest tomatoes growing near infested greenhouses.
Host Plants—The tomato pinworm feeds only on solanaceous plants. Besides tomato, it is common in potato and eggplant. The weeds nightshade and horsenettle are also subject to attack.
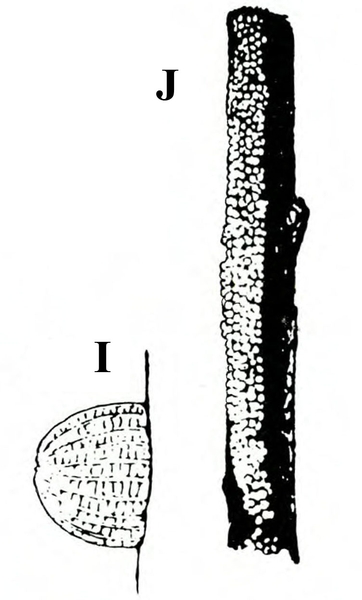
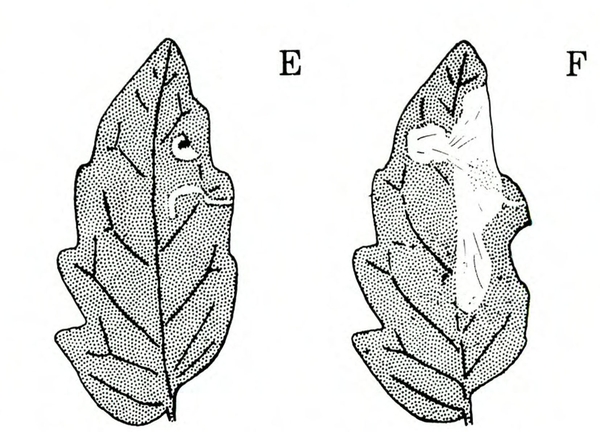
Damage—Pinworms cause blotch-like leaf mines, folded and tied leaves, pinholes in stems and fruit, and blotches on fruit. First- and second-instar larvae mine leaves in a manner similar to that of serpentine and vegetable leafminers; these mines, however, are widened gradually into one large blotch (Figure 26E–F and Figure 27).
Upon emerging from leaf mines, third-instar larvae fold and web leaves for protection and feed inside these shelters. Some of the larvae bore into stems, buds, and fruit, leaving small pinholes on the surface. Larvae usually enter the fruit near calyx lobes or the stem. Larvae rarely penetrate deeper than 3/4 inch and usually feed just below the skin. In addition to pinholes, injured tomato fruits have discolored blotches. Damage to leaves and vines is of little importance, but injury to the fruit can cause a substantial loss.
Life History—In Mexico, California, Florida, and Texas, tomato pinworms overwinter outdoors as pupae at or near the soil surface. In North Carolina and most other states, pinworms spend winter in greenhouses. The nocturnal moths may emerge as early as March or April. Eggs are usually deposited on the undersides of leaves and hatch about one week later. During summer, larvae mine the leaves for about six days and then fold leaves or bore into fruit for another six days. Mature fourth-instar larvae either remain in folded leaves or drop to the soil to pupate. About 12 days later, a new generation of moths emerges.
In summer, a generation can be completed every 26 to 34 days. In cooler weather, the life cycle is longer. Seven to eight overlapping generations occur each year in Florida. It is probable that just as many generations occur in North Carolina greenhouses. If moths escape outdoors, several generations may occur in field tomatoes during summer.
Control
Sanitation and prevention are good control measures for tomato pinworms. Infestations usually result from transplants that are shipped in or grown in local greenhouses. Therefore, close inspection of new plants can prevent serious problems later in the season. For recommended insecticides and rates, consult the North Carolina Agricultural Chemicals Manual.
Vegetable Leafminer
Liriomyza sativae Blanchard, Agromyzidae, DIPTERA
Description
Adult—This shiny, black fly has variable yellow markings and is almost 1/16 inch long (Figure 28A).
Egg—The extremely small, white, oval egg is sometimes visible through the upper epidermis of the leaf via a magnifying glass (Figure 28B).
Larva—The newly hatched larva is nearly colorless and very small (but visible with a magnifying glass). The fully grown maggot, about 1/8 inch long, has a translucent, bright-yellow body and black mouthparts. Each maggot has a slightly pointed head and a rounded abdomen (Figure 28C).
Puparium—The flattened, segmented puparium is bright yellow at first but gradually turns brown. It is oblong-oval in shape and a little more than 1/16 inch long (Figure 28D).
Biology
Distribution—The vegetable leafminer is found from the tropics into the southeastern and southwestern United States. It occurs at least as far north as Tennessee and Ohio. Because this leafminer has long been confused with closely related species, the precise extent of its distribution is not known.
Host Plants—Closely related to the serpentine leafminer that feeds almost exclusively on crucifers, the vegetable leafminer infests a wide variety of plants. In addition to tomato, weed and cultivated crop hosts include squash, okra, pea, bean, cabbage, turnip, potato, tobacco, cotton, radish, spinach, watermelon, beet, pepper, alfalfa, clover, vetch, and plantain.
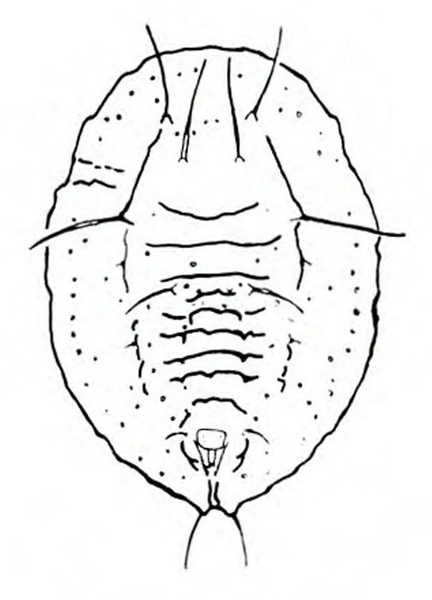
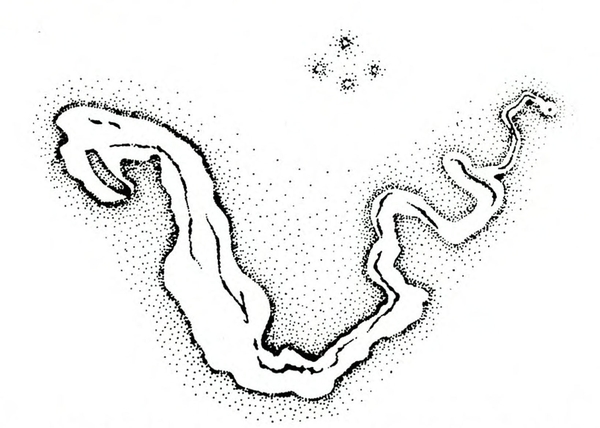
Damage—Like serpentine leafminers, vegetable leafminers create light-colored, irregularly winding mines in leaves. The mines are generally S-shaped and may be enlarged at one end (Figure 28E). Infested leaves are favorable habitats for bacterial and fungal plant pathogens. Also, because heavily mined leaves may have much of their mesophyll (internal tissue) removed, photosynthetic efficiency is greatly reduced (Figure 29).
Severe infestations may cause the foliage to turn brown and appear burned. Damaging infestations are most likely to occur after crops have been treated weekly with insecticides such as methomyl or carbaryl. These pesticides kill parasitic wasps that usually keep the leafminer populations at acceptable levels.
Life History—Vegetable leafminers feed and breed year-round in the southern areas of Florida and Texas. In North Carolina, they overwinter in soil as pupae. Generally, adult flies that emerge in April or May live only four to ten days. After mating, females insert eggs into leaf tissue from the underside of the leaf. Eggs hatch three to eight days later, and young larvae begin feeding, each one creating its own mine. Although the leaf-mining stage may last up to twelve days, it is usually completed in four to five days during summer. Larvae pupate for about 10 days (longer in spring and fall) at the enlarged ends of the mines or in soil. A new generation is produced about every 23 days. At least five generations occur each year in southern states. The number is higher in the tropics and in greenhouses.
Control
On a small scale, it is practical to remove infested tomato leaves to help keep leafminer populations in check. Economically significant leafminer damage rarely occurs on tomatoes. For large-scale producers, insecticides remain the most reliable method of controlling infestations. For recommended insecticides and rates, consult the North Carolina Agricultural Chemicals Manual.
Western Flower Thrips
Frankliniella occidentalis (Pergande), Thripidae, THYSANOPTERA
Description
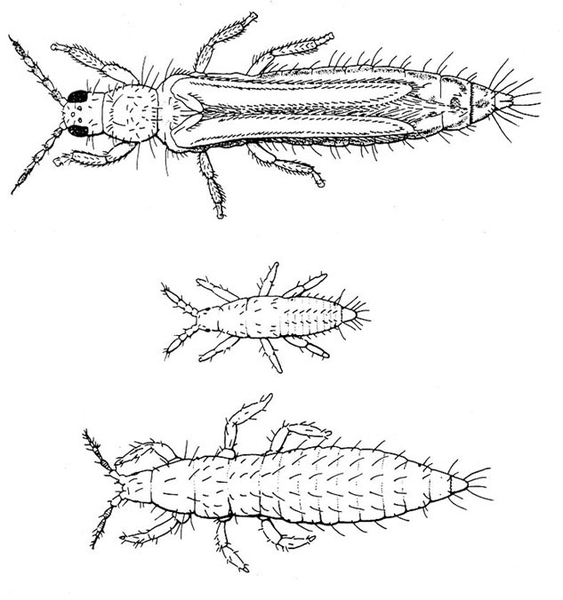
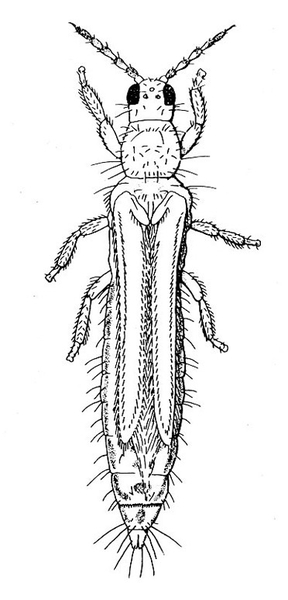
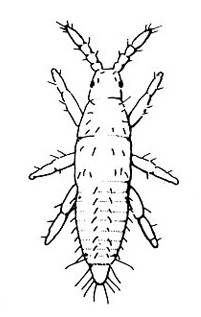
Adult—The western flower thrips (Figure 30A) is almost 1/16 inch long, with the female larger than the male. The female varies from yellow to dark-brown and has a plump abdomen. The male is always pale yellow and has a narrower abdomen.
Egg—Yellowish eggs cannot be seen easily because they are inserted into the plant tissue (Figure 30B).
Larva—The larvae develop through two instars and are distinctly yellow. First instars (Figure 30C) are noticeably smaller than second instars. Second instars (Figure 30D) are about the same size as adult females, and they become whitish before molting.
Prepupa and Pupa—Both prepupa (Figure 30E) and pupa (Figure 30F) are quiescent, nonfeeding stages. They are yellowish. Antennae of prepupae protrude forward, whereas antennae of pupae are folded back over the head. Prepupae have noticeable wing pads on the thorax. Wing pads of pupae extend back along the abdomen.
Biology
Distribution—Before 1980, the distribution was thought to be limited to west of the Mississippi River. However, the western flower thrips has become the most prevalent thrips species attacking greenhouse plants throughout the United States and Canada in addition to many countries in Europe and Asia.
Host Plants—This thrips feeds on almost any flowering plant. Besides tomato, major host plants include carnation, chrysanthemum, gerbera, geranium, marigold, pansy, pepper, and rose.
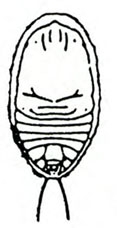
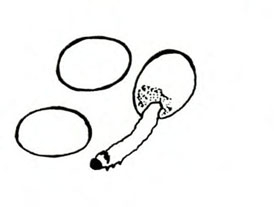
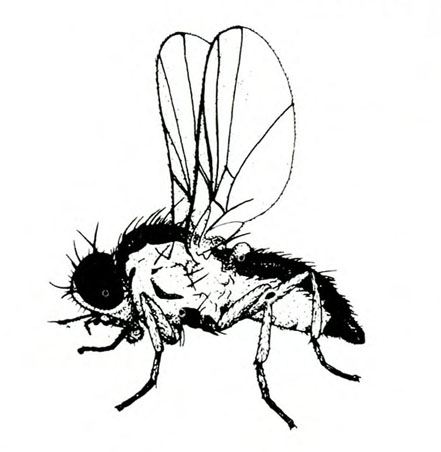
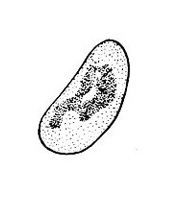
Damage—The western flower thrips feeds on flowers and foliage by inserting its modified left mandible into the tissue, injecting saliva, and sucking the fluids from cells. When thrips feed on developing tissues, affected cells are unable to expand, and maturing leaves and petals become distorted. When thrips feed on expanded tissue, affected cells fill with air, which imparts a silvery appearance. This thrips also is an important vector of tomato spotted wilt virus (Figure 31) and impatiens necrotic spot virus.
Life History—Females lay eggs in tender plant tissue. The eggs hatch in two to fourteen days, depending on temperature. First-instar larvae begin feeding as soon as they hatch. Second-instar larvae also feed on plant tissue, usually in flowers. These larvae are found protected in the perianth of the flower or within developing terminal foliage. Late in the second-instar stage, larvae stop feeding and move down the plant to pupate. Thrips develop through two quiescent, nonfeeding pupal stages in the soil, plant litter, or a protected area on the plant. Adults emerge and resume feeding on flowers, buds, and terminal foliage. The entire life cycle from oviposition to adult emergence can be completed in as little as 12 days in hot weather to as long as 44 days in cool weather.
Control
Thrips are difficult to manage with pesticides because they feed deep in the flowers and buds, where they are sheltered from chemicals. Chemical management of western flower thrips has received much attention by researchers, but control remains difficult. Natural enemies have been investigated, and biological control programs using insidious plant bugs and predaceous mites in the genus Amblyseius have been used in greenhouses. Screening has been shown to effectively exclude western flower thrips. For insecticide recommendations, consult the North Carolina Agricultural Chemicals Manual.
Other Resources
General
Beasley, E. O., et al. Growing Trellised Tomatoes in Western North Carolina. Publication AG-60. North Carolina Agricultural Extension Service, 1977.
Linn, M. B., and J. M. Wright. Tomato Diseases and Insect Pests: Identification and Control. Illinois Agricultural Extension Service, 1951.
Michelbacker, A. E., W. W. Middlekauff, and N. B. Akesson. Caterpillars Destructive to Tomato. Bulletin 707. California Agricultural Experiment Station, 1948.
Cutworms
Busching, M. K., and F. T. Turpin. “Oviposition Preferences of Black Cutworm Moths Among Various Crop Plants, Weeds, and Plant Debris.” Journal of Economic Entomology 69 (1976): 587–90.
Cline, L. D., and D. H. Habeck. “Reproductive Biology of the Granulate Cutworm.” Journal of the Georgia Entomological Society 12 (1977): 34–41.
Harris, C. R., J. H. Mazurek, and G. V. White. “The Life History of the Black Cutworm, Agrotis ipsilon (Hufnagel), Under Controlled Conditions.” The Canadian Entomologist 94 (1962): 1183–87.
Jones, T. H. “The Granulated Cutworm, An Important Enemy of Vegetable Crops in Louisiana.” USDA Bulletin 703 (1918): 7–14.
Rings, R. W., F. J. Arnold, A. J. Keaster, and G. J. Musick. A Worldwide, Annotated Bibliography of the Black Cutworm, Agrotis ipsilon (Hufnagel). Research Circular 198. Ohio Agricultural Research and Development Center, 1974.
Rings, R. W., B. A. Johnson, and F. J. Arnold. A Worldwide, Annotated Bibliography of the Variegated Cutworm, Peridroma saucia Hübner. Research Circular 219. Ohio Agricultural Research and Development Center, 1976.
Snow, J. W., and P. S. Callahan. Biological and Morphological Studies of the Granulate Cutworm, Feltia subterranea (F.), in Georgia and Louisiana. Bulletin 42. Georgia Agricultural Experiment Station, 1968.
Wadley, E. M. “Life History of the Variegated Cutworm.” Journal of Economic Entomology 14 (1921): 272–77.
Greenhouse Whitefly
Antonelli, A., R. Akre, and A. Retan. Whiteflies: Their Biology and Control. EM 3980. Washington State University, College of Agriculture, Cooperative Extension Service, 1979.
Burnett, T. “Aspects of the Interaction Between a Chalcid Parasite and its Aleurodid Host.” Canadian Journal of Zoology 45 (1967): 539–78.
Curry, J. P., and D. Pimentel. “Evaluation of Tomato Varieties for Resistance to Greenhouse Whitefly.” Journal of Economic Entomology 64 (1971a): 1333–34.
Gentile, A. G., R. E. Webb, and A. K. Stoner. “Resistance in Lycopersicon and Solanum to Greenhouse Whiteflies.” Journal of Economic Entomology 61 (1968): 1355–57.
Gerling, D. “Biological Studies on Encarsia formosa (Hymenoptera: Aphelinidae).” Annals of the Entomological Society of America 59 (1966): 142–43.
McClanahan, R. J. Integrated Control of the Greenhouse Whitefly. Canada Department of Agriculture, Information Canada, Ottawa, 1972.
Morrill, A. W. The Greenhouse Whitefly. Circular 57. USDA Bureau of Entomology, 1905.
Russell, L. M. The North American Species of Whiteflies of the Genus Trialeurodes. Miscellaneous Publication 635. U.S. Department of Agriculture, 1948.
Silverleaf Whitefly
Ascerno. M. E. “Knowledge of Which Whitefly Life Stages Are Present at the Time of Treatment Will Help in Making the Best Control Decisions.” GrowerTalks (December 1989): 89.
Bellows, T. S., Jr., T. M. Perring, R. J. Gill, and D. H. Headrick. “Description of a Species of Bemisia (Homoptera: Aleyrodidae). Annals of the Entomological Society of America 87, no. 2 (1994): 195–206.
Prabhaker, N., N. C. Toscano, and D. L. Cooudriet. “Susceptibility of the Immature and Adult Stages of the Sweetpotato Whitefly (Homoptera: Aleyrodidae) to Selected Insecticides.” Journal of Economic Entomology 82 (1989): 983–88.
Price, J. D., J. Schuster, and J. B. Kring. “A Comprehensive View of the Sweetpotato Whitefly and Its Management in Ornamental Plants.” In Proceedings of the 5th Conference on Insect and Disease Management on Ornamentals, 19–23, Feb. 26–28, 1989, Orlando, FL.
Gerling. D., ed. “Natural Enemies of Whiteflies: Predators and Parasitoids.” In Whiteflies: Their Bionomics. Pest Status and Management, 147–85. Newcastle upon Tyne, England: Athenaeum Press, 1990.
Tobacco Budworm
Capinera, J. L. Common Name: Tobacco Budworm, Scientific Name: Heliothis virescens (Fabricius) (Insecta: Lepidoptera: Noctuidae). Featured Creatures. Publication Number: EENY–219. Entomology and Nematology, FDACS/DPI, EDIS, UF/IFAS, University of Florida, 2018.
Tomato Fruitworm
Canerday, T. D. Tomato Fruitworm Control. Leaflet 74. Alabama Agricultural Experiment Station, 1967.
Marcovitch. S., and W. W. Stanley. The Tomato Fruitworm in Tennessee. Bulletin 174. Tennessee Agricultural Experiment Station, 1941.
Wilcox, J., A. F. Hawland, and R. E. Campbell. Investigations of the Tomato Fruitworm, Its Seasonal History, and Methods of Control. Technical Bulletin 1147. USDA, 1956.
Wilcox, J., and A. F. Howland. The Tomato Fruitworm: How to Control It. Leaflet 367. USDA, 1972.
Tomato Hornworm
Garman, H., and H. H. Jewett. The Broods of the Tobacco Worms. Bulletin 225. Kentucky Agricultural Experiment Station, University of Kentucky, 1920.
Gilmore, J. U. “Observations on the Hornworms Attacking Tobacco in Tennessee and Kentucky.” Journal of Economic Entomology 31 (1938): 706–12.
Tomato Pinworm
Batiste, W. C., and W. H. Olson. “Laboratory Evaluations of Some Solanaceous Plants as Possible Hosts for Tomato Pinworm.” Journal of Economic Entomology 66 (1973): 109–11.
Elmore, J. C. The Tomato Pinworm. Circular 440. USDA, 1937.
Elmore, J. C., and A. F. Howland. Life History and Control of the Tomato Pinworm. Technical Bulletin 841. USDA, 1943.
Poe, S. L. Common Name: Tomato Pinworm, Scientific name: Keiferia lycopersicella (Walshingham) (Insecta: Lepidoptera: Gelechiidae). Featured Creatures. Publication Number: EENY–74. Entomology and Nematology, FDACS/DPI, EDIS, University of Florida, 2020.
Vegetable Leafminer
Musgrave, C. A., S. L. Poe, and H. V. Weems, Jr. The Vegetable Leafminer, Liriomyza sativa Blanchard (DIPTERA: Agromyzidae), in Florida. Entomology Circular 162. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, 1975.
Spencer, K. A. “Agromyzidae (Diptera) of Economic Importance.” Series Entomologica 9 (1973).
Spencer, K. A., and C. E. Stegmaier, Jr. Agromyzidae of Florida with a Supplement on Species from the Caribbean. Gainesville, FL: Florida Department of Agriculture and Consumer Services, Division of Plant Industry, 1973.
Stegmaier, Jr., C. E. “Host Plants and Parasites of Liriomyza munda in Florida (DIPTERA: Agromyzidae).” Florida Entomologist 49 (1966): 81–86.
Stegmaier, Jr., C. E. “A Review of Recent Literature on the Host Plant Range of the Genus Liriomyza Mik. (Diptera: Agromyzidae) in the Continental United States and Hawaii, Excluding Alaska.” Florida Entomologist 51 (1968): 167–82.
Steyskal, G. C. “The Strange Fate of the ‘Serpentine Leafminer’ (Liriomyza spp., Agromyzidae, DIPTERA).” USDA Cooperative Economic Insect Report 23, no. 43 (1973): 735–36.
Webster, R. M., and T. H. Parks. “The Serpentine Leaf-miner.” Journal of Agricultural Research 1 (1913): 59–88.
Wolfenbarger, D. O., and D. A. Wolfenbarger. “Tomato Yields and Leafminer Infestations and a Sequential Sampling Plan for Determining Need for Control Treatments.” Journal of Economic Entomology 59 (1966): 279–83.
Western Flower Thrips
Bryan, D. E., and R. F. Smith. “Frankliniella occidentalis Complex in California.” The University of California Publications in Entomology 10, no. 6 (1956): 359–410.
Higgins, C. J., and J. H. Myers. “Sex Ration Patterns and Population Dynamics of Western Flower Thrips (Thysanoptera: Thripidae).” Environmental Entomology 21, no. 2 (1992): 322–30.
Robb, K. L. “Analysis of Frankliniella occidentalis (Pergande) as a Pest of Floricultural Crops in California Greenhouses.” Ph.D. Dissertation, University of California, Riverside, 1989.
Ullman, D. E., et al. “Internal Morphology of Frankliniella occidentalis (Pergande) with Special Reference to Thrips Interactions Between Thrips and Tomato Spotted Wilt Virus.” International Journal of Insect Morphology and Embryology 18, nos. 5–6 (1989): 289–310.
Publication date: June 17, 2024
AG-295
Other Publications in Insect and Related Pests of Vegetables
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.