Introduction
Vegetable crops are grown in a range of settings in North Carolina, both commercial and residential. They may be cultivated in large fields, small plots, and large or small greenhouses. Commercial vegetable crop production in the state requires one of the highest dollar-per-acre investments of all agricultural endeavors, which makes it extremely important to prevent insects from reducing yields, lowering quality, and increasing production costs. The lessening of just one market grade can be economically devastating for the producer. Home gardeners also have much at stake, as they are investing more in growing vegetables. Interest in home gardening is at its highest level since the trend of planting Victory Gardens during the World War II era. The incentives for growing vegetables at home include increased grocery prices and better quality and freshness of home-grown produce.
Whether vegetable crops are grown in commercial fields, home gardens, or greenhouses, the first step in alleviating pest damage is accurate identification of the culprit. Growers should educate themselves about pest biology and the types of pests that attack crops in their locale. Such knowledge will enable the proper implementation of management and control practices—informing scouting methods, equipment selection, timing of controls, and other pest management practices. Not all insects and mites threaten plants, so a suspected pest may prove to be harmless or even beneficial. Strive to avoid mistaking insect damage for injuries caused by disease, drought, or fertilizer misuse.
The purpose of this publication is to give growers the necessary information to identify the important, common pests of vegetables grown in North Carolina. Each section is dedicated to a specific vegetable crop, beginning with descriptions of physical characteristics of their typical pests and indicators of their presence, including illustrations, followed by individual profiles of some of the major pest organisms for that crop. The profiles include a description of the life stages, distribution, host plants, signs of damage, life history, and control methods.
Following are descriptions of the two broad categories of pest control—nonchemical and chemical. A separate section applicable to greenhouse production follows.
Nonchemical Control
Nonchemical pest control in a garden involves prevention and treatment—including cultural, physical, mechanical, and biological practices. Nonchemical pest management strategies include preventive practices such as planting resistant varieties ("cultivars"), certified seeds, and pest-free transplants; rotating crops; controlling weeds and sanitizing; using mechanical barriers; hand removal or organic treatment when pests occur; and encouraging the presence of beneficial parasites, predators, and diseases.
The success of nonchemical control depends largely on economics, goals of the grower, and the growing environment. Large-scale commercial vegetable production almost always requires the use of pesticides, whereas a home gardener with fewer plants to manage may rely primarily on nonchemical methods. This does not imply that nonchemical controls are only for home gardeners and organic farmers; they may be employed in greenhouses or conventional commercial field crops in combination with traditional pesticides.
The grower who chooses the organic method of pest control (produced without synthetic chemicals) should keep in mind several factors. Vegetables produced organically may be slightly to heavily damaged by pests. With organic gardening, more labor is required in controlling pests. Damage from soil pests is to be expected. To compensate for anticipated losses, larger gardens should be planted so there will be enough food for both gardener and insects. Susceptible crop cultivars should be avoided.
Following is a brief overview of nonchemical growing strategies.
Healthy Plant Selection—The primary nonchemical defense against pests begins outside the garden with the selection of hardy seeds and plants.
-
Choose resistant varieties or cultivars that repel, are unattractive to, or are otherwise unsuitable as food for certain insects or that withstand feeding by certain pests with little reduction in yield or quality. Using resistant cultivars fits well into just about any production schedule. A factor to consider, however, is whether the harvested crop will be as desirable (as large or palatable) as comparable nonresistant cultivars. The grower should weigh the potential for pest damage against the desired quality or type of produce. Cultivars adapted to weedy conditions should also be considered.
-
Plant certified seeds that are guaranteed to have a certain percent germination and to be virtually free of weed seed. The use of certified seed or pest-free plants, when available, enables the grower to start with healthy, vigorous stock. Such plants—though not necessarily pest-resistant—have a good chance of withstanding attack.
-
Use disease-free transplants. If you are producing your own transplants, raise them under healthy conditions. Proper management practices can effectively control insects in the plant bed. Sanitation, adequate seedbed preparation, isolation of plant beds from infested areas, and frequent, thorough examination of plants for initial infestations and prompt treatment will favor successful plant establishment and minimize pest problems. Growers should inspect new transplants carefully for pests such as pepper weevil, tomato pinworm, and sweetpotato weevil before transplanting. Tomato pinworm and pepper weevil are examples of serious pests that are brought into North Carolina, primarily on transplants from Florida.
Crop Rotation—Many vegetable crops are closely related biologically and share the same pests. Insects that become severe problems on cabbage, for example, are likely to infest nearby crucifers like mustard, turnip, broccoli, collard, or cauliflower. Therefore, it is often advisable to plant closely related crops as far from each other as possible and to plant them in a different place ("rotate" them) in subsequent years.
Cultivation, Weed Control, and Sanitation—These three practices are interrelated. Cultivation can be used to control weeds, to bury or destroy crop residue, or to expose soil pests to the elements. Sanitation includes the removal and destruction of dead or diseased plants; because some insects hasten the spread of bacterial and viral plant diseases, the sooner these plants are removed, the better.
Mechanical Barriers—Collars made of cardboard, tin cans (with tops and bottoms removed), or aluminum foil are effective barriers against cutworms. When placed around seedlings and inserted into the soil, collars prevent cutworms from moving through the upper soil to seedling stems. This method is most practical in small gardens. Other examples of mechanical barriers include row crop covers over collard plants (to exclude egg-laying diamondback moths) and screened storage areas for potatoes (to exclude potato tuberworm moths).
Hand Removal—Removing and destroying pests by hand is particularly effective in small gardens. If contact with pests is repugnant to the gardener, pests can be knocked into soapy water or onto the ground and stepped on. Removal of egg masses or leaves with egg masses can also be beneficial.
Traps—Insect traps are used to survey, detect, and manage pests. Traps are commonly used in large-scale commercial production as gauges of potential infestations. Certain traps are useful in small gardens. Some insects, such as squash bugs, will congregate under strategically placed boards. By regularly checking beneath the boards, gardeners can trap and kill a number of pests at once.
Beneficial Parasites, Predators, and Diseases—Beneficial organisms may naturally inhabit gardens and vegetable fields, helping keep pests in check. These organisms can be purchased, but establishing them in new environments may be difficult. Parasites of the greenhouse whitefly are available commercially and are effective. However, introduced arthropods—such as lady beetles—often leave if there are not sufficient numbers of pests for them to consume. Therefore, it is best to learn to recognize the naturally occurring beneficial organisms already present and to work to maintain their populations by preserving their habitat needs. Keep in mind that the use of synthetic organic pesticides is detrimental to many beneficial insects and mites. Insect disease organisms—including many viral, fungal, bacterial, and nematode pathogens—also lower pest numbers. Bacillus thuringiensis (Bt), for example, is a widely known bacterial insecticide that is effectively used to control young caterpillar populations. This bacterium is discussed under Chemical Control.
Chemical Control
This manual is designed to augment the North Carolina Agricultural Chemicals Manual, which is updated annually. Always consult that publication for instructions on current proper selection and safe application of chemicals.
Pesticide Mechanisms—Insect feeding habits determine, to some extent, the type of chemical control used. Pesticides can be classified as stomach poisons (such as carbaryl), contact residuals (such as malathion and pyrethroids), systemics (such as imidacloprid), and fumigants. All types are used in vegetable production. Note that fumigants are used primarily in greenhouses; these are discussed in the subsequent section, Pest Control in Greenhouses.
Stomach poisons target caterpillars, beetles, grasshoppers, and other chewing insects. The diverse array of botanical, organic, and inorganic chemicals that make up this type of pesticide must be ingested for pests to die. Because piercing-sucking pests (aphids, mites, leafhoppers, and whiteflies) feed on sap below the plant surface rather than consuming insecticide-contaminated tissue, they may not come in sufficient contact with stomach-poison pesticides to be killed.
Contact-residual chemicals are applied to the entire plant; therefore, they kill a wider range of pests, including insects or mites that crawl on treated surfaces, eat treated leaves, or are sprayed directly. Summer or dormant oils are a type of contact residual that kills by disrupting the integument of tiny insects or mites, causing them to become dehydrated.
Bacillus thuringiensis (Bt) is the active ingredient of Dipel, Sok BT, Bactospeine, and Thuricide. Safe to humans, animals, and beneficial insects, it is a very selective pesticide. Only moth and butterfly caterpillars are affected by Bt spores, primarily when the caterpillars are young.
Efficacy—The persistence of a pesticide after it has been applied depends to some extent on the chemical group to which it belongs. Materials like the plant-derived pesticide pyrethrum last less than one hour, although similar synthetic pyrethroid pesticides that last considerably longer are now available. In general, insecticides classified as chlorinated hydrocarbons are long-lasting, although some break down fairly rapidly. Less persistent than chlorinated hydrocarbons, most organophosphates and carbamates remain effective from only one to four weeks. Although all classes of pesticides are used to control vegetable pests, most of the chemicals recommended for pest control on vegetables must be relatively short-lived so that produce will be safe to eat. Federal law limits the levels of insecticides and fungicides that can remain as residues in or on raw agricultural crops. These residue limits are known as tolerances. By adhering to manufacturer's directions, growers are assured of harvesting produce that will meet tolerances established by the U.S. Food and Drug Administration (FDA). Any market-bound produce that exceeds established tolerances is subject to confiscation.
The effectiveness of a pesticide depends on more than how it chemically affects the target organism. For example, the timing of application is also key to success—not just the time of day or the weather conditions under which they are applied, but also the current life stage of the pests at the time of application and their level of abundance. Pesticides must be applied carefully and at the proper rates, all of which should be provided with the label instructions. It is wise to keep a close watch on the performance of an insecticide. Should increasing ineffectiveness be caused by development of pest resistance, other pesticide materials can be substituted. Frequent substitution substantially reduces the chances that pests will develop resistance. Most ineffectiveness, however, is due to poor application techniques.
Choosing Pesticides—Amateur gardeners must choose from pesticides labeled for home use. The home gardener can lessen the need for purchasing additional pesticides later by initially selecting a product that can be used on a wide variety of crops and against many pests. To eliminate long-term storage responsibility, it is a good idea to buy quantities small enough to be used in one growing season. Since most homegrown vegetables go straight from the garden to the table, choosing an insecticide with a brief prescribed waiting period between application and harvest is advisable. A broad range of chemicals of many kinds and formulations are cleared for use on commercially produced vegetables. Soil-applied granules and fumigants, foliar sprays, and systemics all have a place in commercial vegetable production.
Monitoring—Scouting for pests has become a common control practice in commercial vegetable production. Checking fields every two or three days for pests, damaged foliage, eggs, and frass (excrement) keeps the grower aware of the status of pest populations. Regular scouting, along with trap surveys, can provide early signals of building infestations. For some insects, such as soil pests, it is not possible to forecast outbreaks. Such pests, which are likely to recur yearly, need to be controlled routinely so that damaging infestations will not have a chance to develop.
Application Timing Tips—In general, calm periods of the day, especially early morning and evening, are optimum times to apply pesticides and minimize drift. Because many broad-spectrum chemicals are used in controlling vegetable insects, pesticide drift poses a particular threat to bees. Spraying around noon is not recommended, even on calm days, because bright sunlight on spray droplets may injure foliage. Sometimes pesticides are best applied with additives; for example, wetting agents may be needed to keep sprays from running off of plants with waxy leaves, such as cabbage and other cole crops.
Equipment and Maintenance—Many types of applicators are available to amateur gardeners. Dusters dispense pesticides in a form that requires no mixing. Though not as efficient as sprayers, dusters offer quick, convenient, visible application. To prevent excessive drift, apply dust when there is little or no wind.
Hose-end sprayers use water pressure to dilute and deliver pesticides to the target pest. Concentrated pesticide is placed in the hose-end sprayer and partially diluted; then water pressure siphons, dilutes, and propels the pesticide to the desired area. Length of the hose limits the range of the hose-end sprayer. Other sprayers use air pressure to force diluted pesticides from a nozzle that has spray-pattern options ranging from very fine to coarse. For the large garden, electric and gasoline powered sprayers are available.
Care of spray equipment is not difficult. As long as a duster is kept dry, the only maintenance required is placing an occasional drop of oil on the plunger rod or other moving parts. Although some sprayers are fitted with strong, plastic parts that do not corrode, rinse them after using. Also after each use, wash sprayers with metal tanks (even stainless steel) three times with clear water to prevent corrosion. For metal sprayer tanks, use about 1 tablespoon of household ammonia (shaken thoroughly) to neutralize corrosive effects of any insecticide residue and prolong the life of the tank. Allow the tank to dry completely. Lightly oil the plunger rod. Don't use herbicides and insecticides in the same sprayer.
Before the growing season and frequently throughout, commercial producers should calibrate equipment, clean and adjust nozzles, and replace defective parts. Spraying with improperly adjusted equipment wastes time, money, and energy; it can also result in poor pest control, crop injury, or illegal insecticide residue at harvest.
Pest Control in Greenhouses
Growing crops in greenhouses protects them from the elements but can also provide shelter for pests. In general, insects, mites, and slugs reproduce more rapidly in warmer temperatures present in greenhouses. Nonchemical control methods include weeding inside and outside of the greenhouse to reduce alternate hosts for vegetable plant pests. Screening doors and vents makes it harder for moths and beetles to fly in and lay eggs or feed. As careful as the grower may be, however, sooner or later an insect or mite will come in on the clothes of workers—or on introduced plants, in pots, or in soil—and threaten a crop. Application of pesticides is important to most growers producing greenhouse crops.
Many greenhouse growers apply pesticides on a periodic, prophylactic basis as insurance against accidental infestations. However, applying pesticides based on proper monitoring and periodic inspection is a better control strategy. Nonselective application of pesticides may reduce not only greenhouse pests, but also their parasites and predators. Pests tend to be more resistant to pesticides than their predators and parasites, so beneficial organisms may be disproportionately harmed. Growers should check plants daily or every other day for insects, mites, or slugs and apply appropriate pesticides.
Pesticides are applied in greenhouses via aerosols, mists, smokes, fogs, dusts, sprays, drenches, and granules. Aerosols, smokes, mists, and fogs should be applied when the greenhouse is closed (at night or in the winter). Place smoke fumigators so that the smoke does not vent directly onto the plant foliage. Because most pesticides are sensitive to ultraviolet rays, the later in the evening a treatment is applied, the more effective it will be. Some growers who use smoke fumigators light those farthest from the door first, which allows the growers to vacate the house before it becomes filled with dangerous fumes. Two people should always be present when applying toxic substances in the greenhouse. If one person gets accidentally poisoned, the other can move that person to safety and call for help. After treatment with fogs, smokes, or aerosols, the house must be ventilated before workers can safely re-enter.
Apply dusts and sprays with conventional dusting or spraying equipment. Apply granular insecticides with a hand-held shaker or some other device that does not grind the granules. Do not handle the plants or potting mix until the granular pesticide has been washed from the foliage and watered in thoroughly.
Keys to Kinds of Insects and Related Pests
This section contains four keys: one for identifying adult pests and three for identifying immature stages. In general, the adult stages are easiest to identify, especially in winged insects. However, it can be tricky to determine if a nonwinged species is mature or immature. Follow these truisms: If a pest has wings or is mating, laying eggs, or giving birth to young, it is an adult. Otherwise, you can consider the pest an immature specimen and identify it using the “Keys to Immature Stages.”
For help with pest identification or disease diagnosis, you may contact your local N.C. Cooperative Extension center. Extension agents have been trained in how to properly handle insect and plant specimens for diagnosis. Some counties have diagnostic plant clinics. North Carolina State University houses a Plant Disease and Insect Clinic to help growers diagnose insect and plant problems. Once a pest has been identified, your Extension agent or specialist may recommend a pesticide or other control measure. You can also consult the North Carolina Agricultural Chemicals Manual.
Key to Adults
-
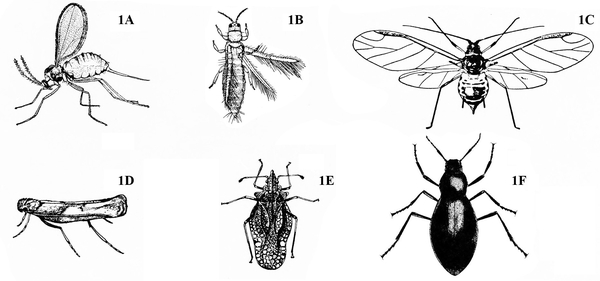
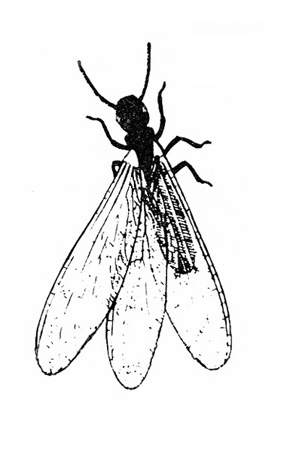
Wings present (Figure 1A–F) — GO TO 2.
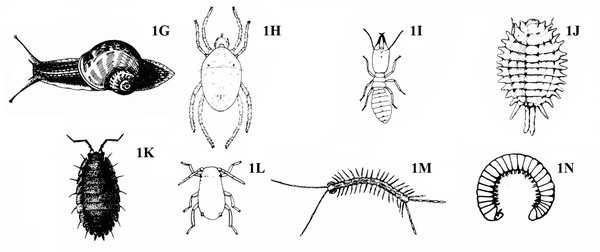
Wingless (Figure 1G-N) — GO TO 10. -
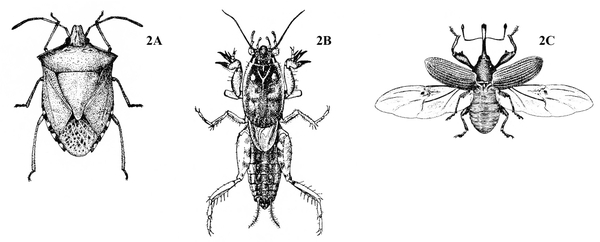
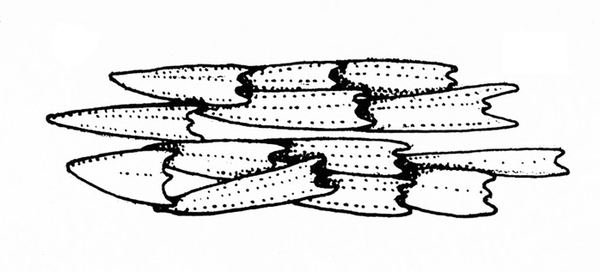
Front pair of wings (the wings that lie on top when folded) partially or completely thickened and leathery (Figure 2A–C) — GO TO 3.
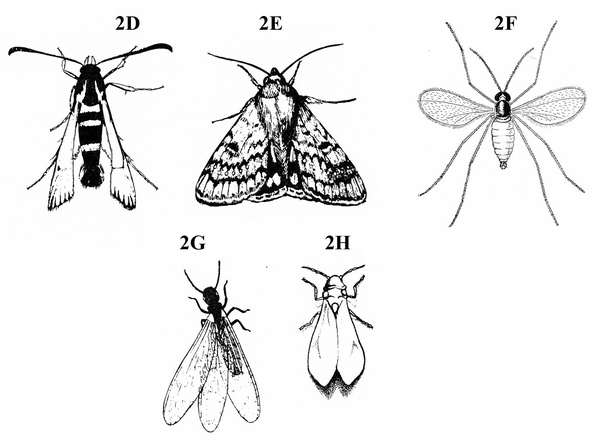
Front pair of wings flexible and papery, sometimes clear (Figure 2D–H) — GO TO 5. -
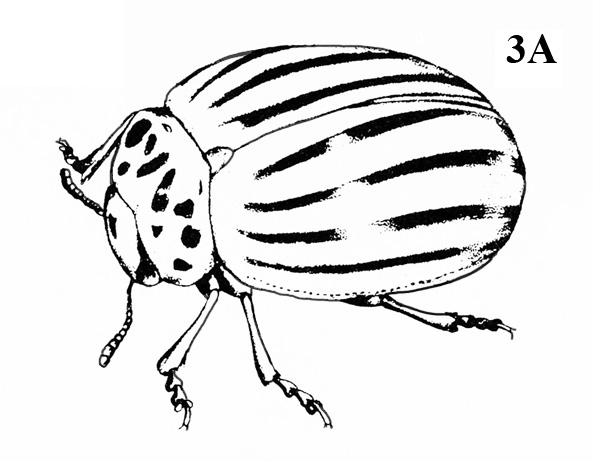
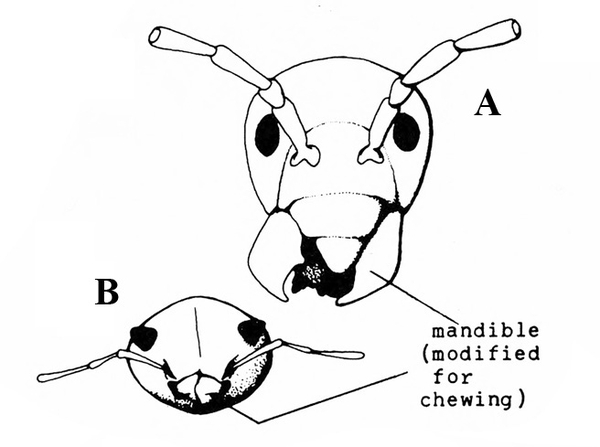
Front pair of wings usually hard, thick, opaque, and lacking veins (Figure 3); mouthparts chewing type (Figure 4A–B) — BEETLES
Front pair of wings usually leathery, with veins (Figure 6); mouthparts chewing type (Figure 5) or extended into a tube (Figure 7) — GO TO 4. -
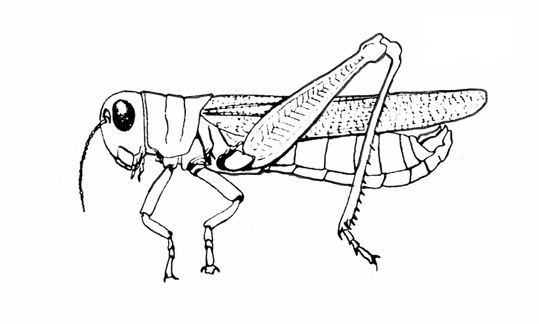
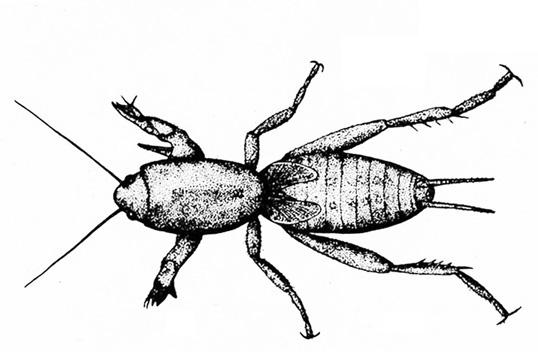
Mouthparts chewing type (Figure 5); hind legs adapted for jumping (Figure 6) or front legs modified for digging (Figure 2B) — GRASSHOPPERS, MOLE CRICKETS.
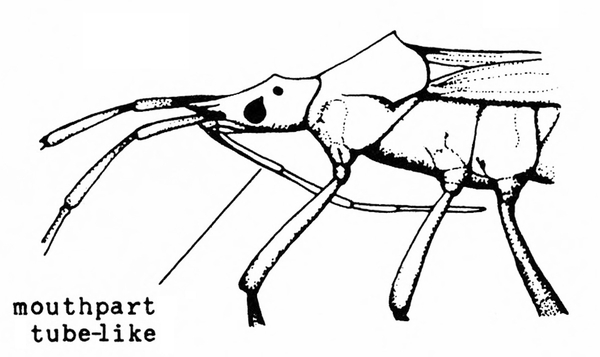
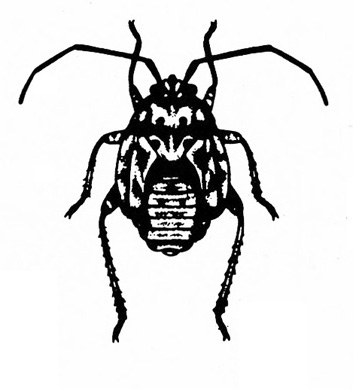
Mouthparts extended into a tube (Figure 7); hind legs usually not adapted for jumping — BUGS
-
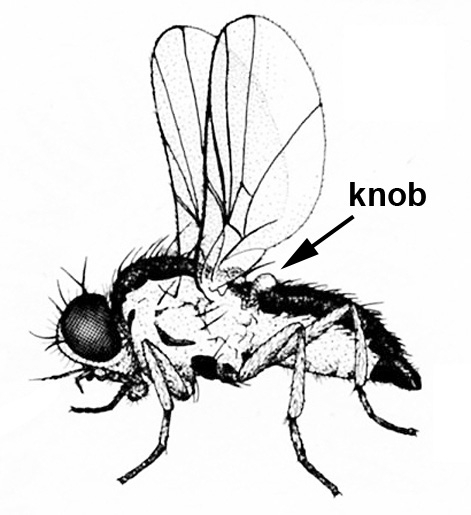
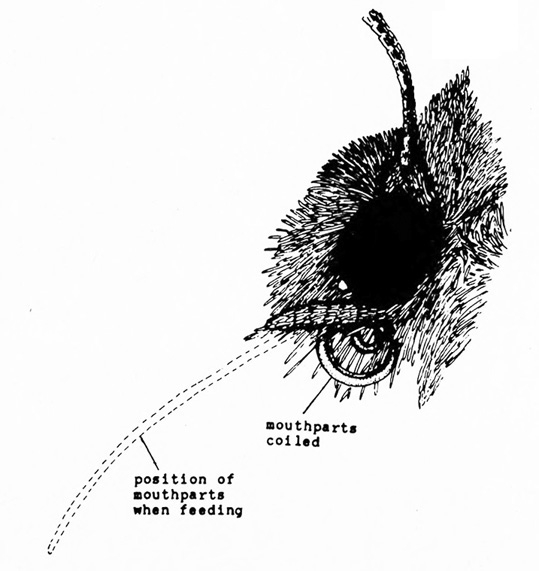
Only one pair of wings present, usually clear (Figure 8); mouthparts adapted for sponging or sucking; second pair of vestigial wings represented by small knobs (Figure 8) — FLIES
Two pairs of wings present (Figure 10 and Figure 11); mouthparts other than sponging type — GO TO 6.
-
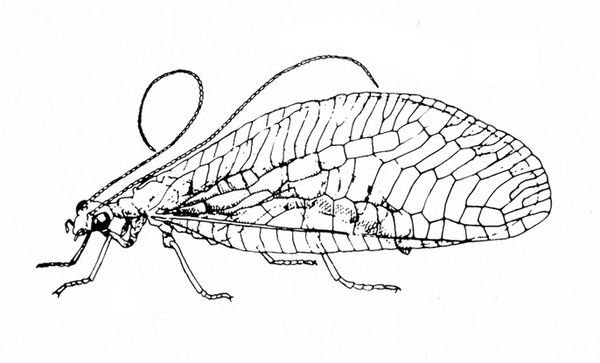
Mouthparts chewing type (Figure 4A–B and Figure 5); wings with network of light, tiny veins evenly covering surface; front wings similar in size to hind wings; fragile insects; antennae filiform (Figure 10 and Figure 11) — GO TO 7.
Mouthparts extended into tube or hairlike structure, or not chewing type (Figure 7 and Figure 9); hind wings usually smaller than front wings; antennae variable. — GO TO 8. -
Wings with many cross veins and branches of veins; insect usually solitary; tarsi five-segmented (Figure 10) — LACEWINGS AND RELATIVES
Wings with reduced venation, but having veinlike wrinkles (Figure 11); insect usually in colony; tarsi four-segmented — TERMITES -
Wings covered with tiny scales that resemble dust when smudged with a finger (Figure 12); mouthparts long, threadlike (Figure 9) — BUTTERFLIES AND MOTHS
Wings without scales; mouthparts variable or lacking — GO TO 9. -
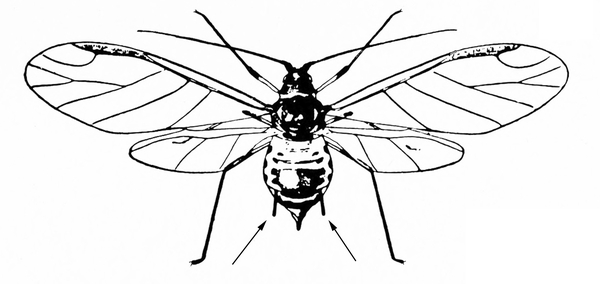
Body with cornicles (tailpipe-like appendages); slow-moving insects; seem to reproduce rapidly (Figure 13) — APHIDS
Body without cornicles — GO TO 14. -
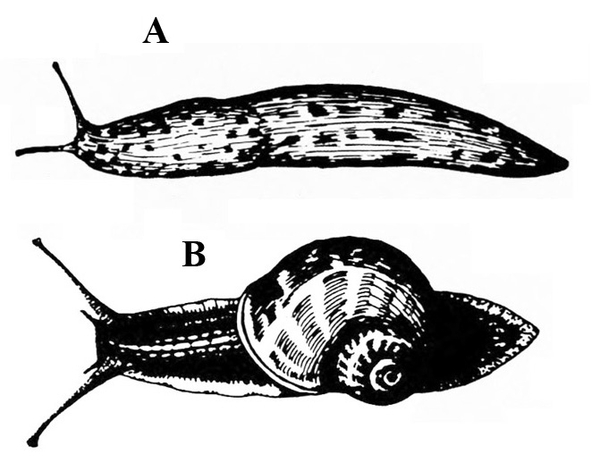
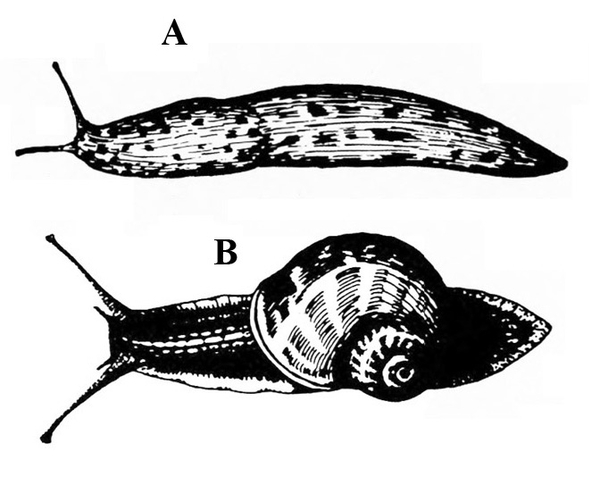
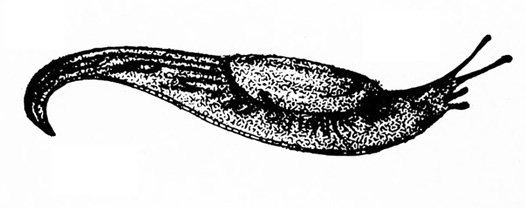
No legs; soft, slimy (slug, Figure 14A), sometimes with a helical shell (snail, Figure 14B)— SLUGS, SNAILS
Legs present — GO TO 11. -
More than or less than six legs present (Figure 15A–C) — GO TO 12.
Six legs usually present; legs and antennae well developed; body with cornicles; mobile (Figure 16) — APHIDS -
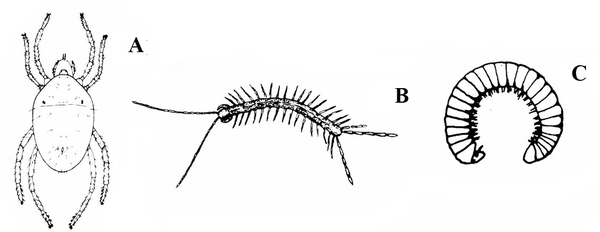
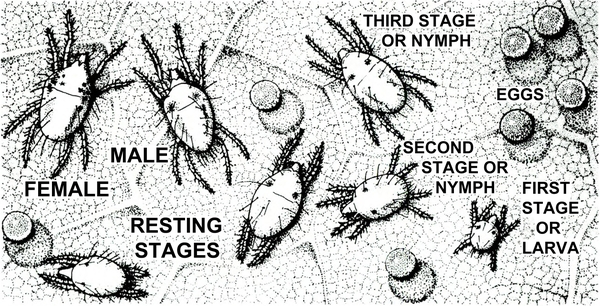
Four pairs of legs present; usually associated with chlorotic stippling of host plant leaves; tiny silk "spider webs" on heavily infested plant; chlorotic stippling symptoms developing rapidly; legs arranged somewhat like a typical spider; color variable (Figure 17) — SPIDER MITES
More than four pairs of legs present— GO TO 13. -
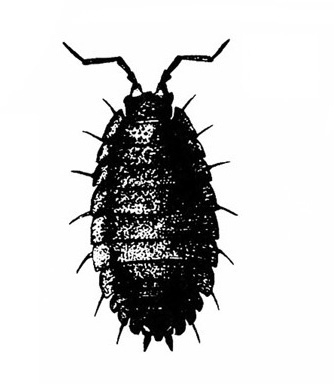
Seven pairs of legs present; oval; sometimes capable of rolling up into a ball (Figure 18) — SOWBUGS, PILLBUGS
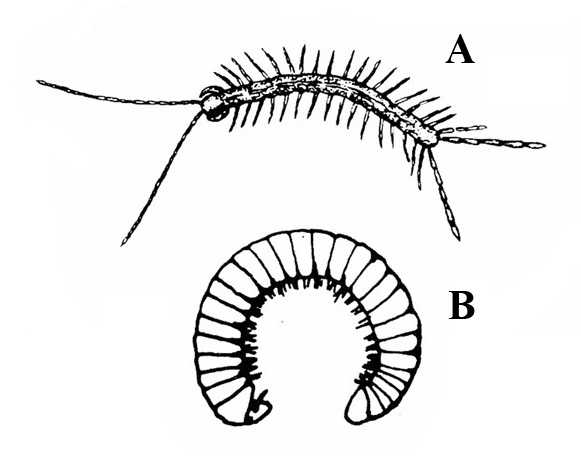
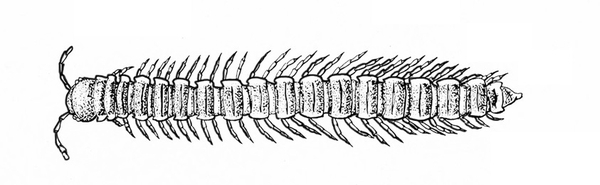
Many pairs of legs present; sides straight; long, slender body sometimes coiling into a helix (Figure 19A–B) — CENTIPEDES, MILLIPEDES -
Small insects (1/16 inch or less); run or flutter when disturbed — GO TO 15.
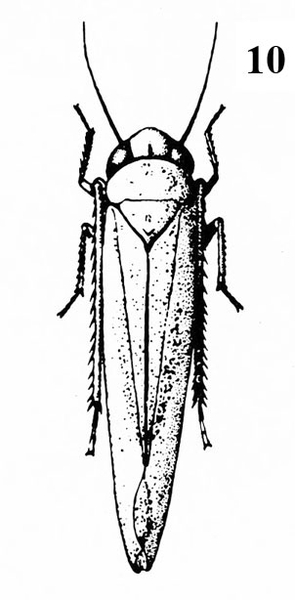
Slightly larger insects (1/16 to 3/8 inch); jump when disturbed (Figure 20) —LEAFHOPPERS -
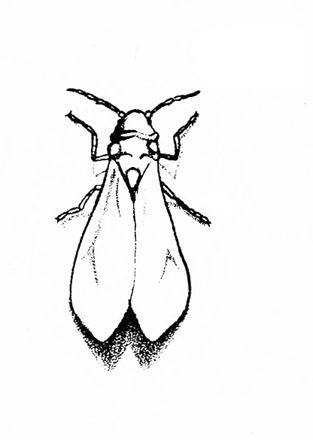
White insects (up to 1/16 inch) that resemble tiny moths; often found on the undersides of host plant leaves; often associated with honeydew and sooty molds; flutter when disturbed (Figure 21) — WHITEFLIES
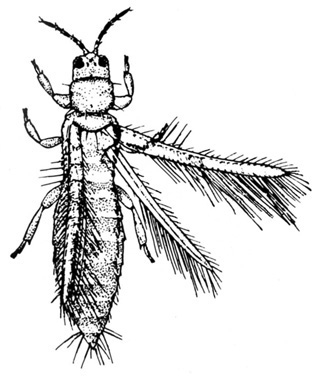
Orange, brown, or black insects (up to 1/16 inch) that are slender and spindle-shaped; often found in buds or flowers, foliage, and even corms; often associated with chlorosis and distorted growth; run or fly when disturbed (Figure 22) — THRIPS
Keys to Immature Stages
Blossom, Leaf, and Fruit Feeders
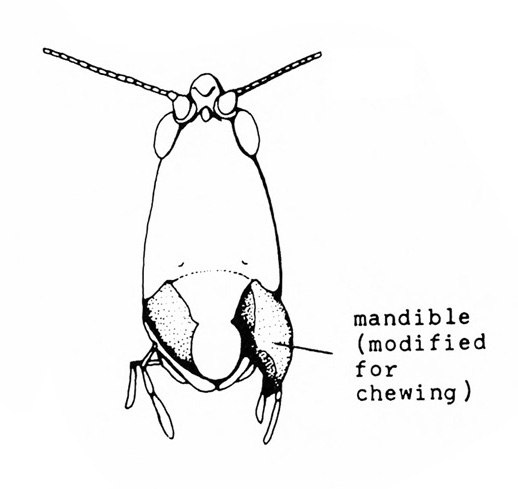
- Chewing mouthparts (leaf removed or consumed by pest) (Figure 4 and Figure 5) — GO TO 2.
Mouthparts extended into a tube or hairlike structure (Figure 7 and Figure 9); leaf may be distorted or discolored but not consumed by pest — GO TO 11. - Insect inside leaf, fruit, pod, or stem. — GO TO 3.
Pest on exterior of plant — GO TO 8. -
Insect mining in foliage — GO TO 4.
Insect feeding inside fruit or pod — GO TO 5. -
Legless insect making mines in leaves — LEAFMINING MAGGOTS
Insect mining in leaves and later hiding in rolled-up leaves; has three pairs of legs and four pairs of prolegs (Figure 23) — LEAFMINING CATERPILLARS -
Larva legless — GO TO 6.
Larva with legs — GO TO 7. -
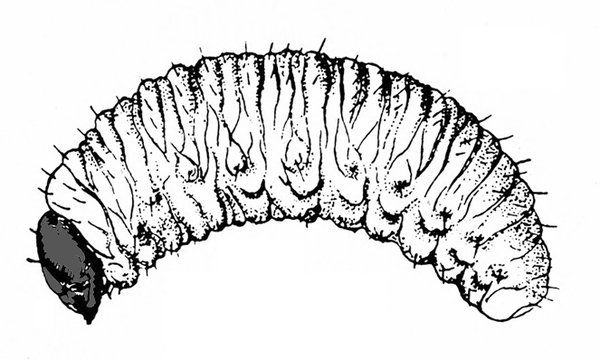
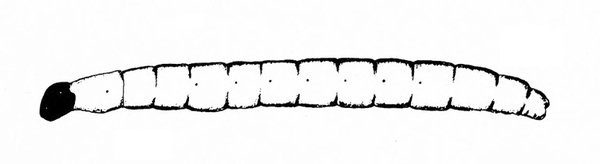
Fairly hard-bodied larva; yellow or white; slender and roughly cylindrical with pointed head and rounded abdomen (Figure 24) — MAGGOTS
Fairly soft-bodied larva; color variable, usually white, grayish white, or pale yellow with dark head; body somewhat curved or C-shaped; lacks legs (Figure 25) — WEEVIL LARVAE -
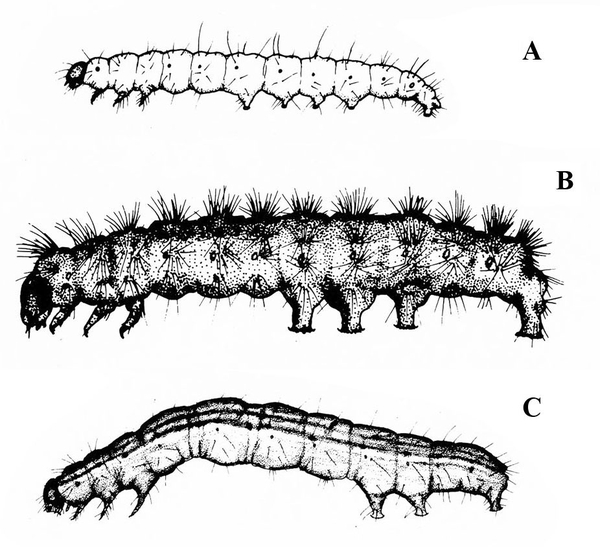
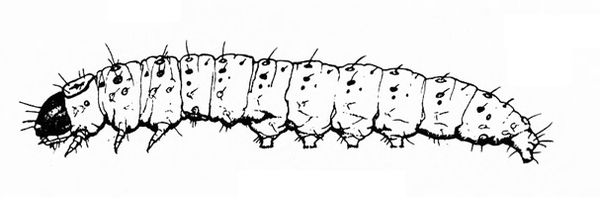
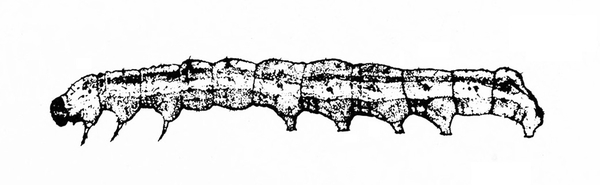
Larva with three pairs of legs near the head and five, four, or three pairs of prolegs (Figure 26A–C) — MOTH CATERPILLARS
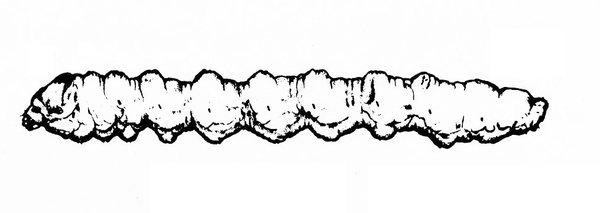
Larva with three pairs of legs near the head and one pair of anal prolegs (Figure 27A–C) — BEETLE LARVAE -
Legless larva — GO TO 9.
Larva with legs — GO TO 10. -
Slime trail often noticed on damaged portion of plant; dark-colored, soft-bodied, slimy animal, sometimes with a helical shell (Figure 28A–B) — SLUGS AND SNAILS
Fairly soft-bodied larva; color variable, usually pale with dark head; body somewhat curved or C-shaped; lacks legs (Figure 29) — WEEVIL GRUBS -
Larva with three pairs of legs near the head and at least three pairs of fleshy prolegs on the abdomen (Figure 30A–C) — MOTH OR BUTTERFLY CATERPILLARS
Larva with three pairs of legs near the head; either one pair of prolegs at the tip of the abdomen or no prolegs (Figure 31A–C) — BEETLE LARVAE -
Pest mobile, with cornicles on the abdomen and sometimes a tail-like cauda (Figure 32A–B) — APHID NYMPHS
Mobility variable; no cornicles or cauda on abdomen — GO TO 12. -
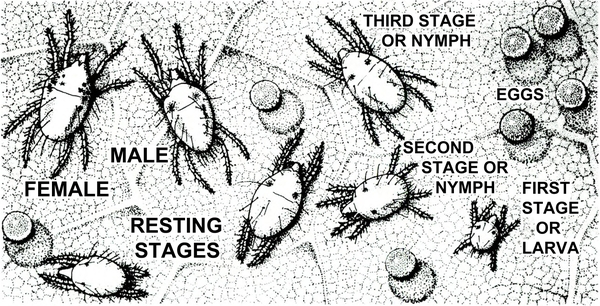
Almost microscopic; three or four pairs of legs; usually associated with very fine webbing, spherical eggs, chlorotic stippling of host plant, and adult spider mites (Figure 33) — SPIDER MITE LARVA, NYMPHS, and RESTING STAGES
Not as above — GO TO 13. -
Very small, active, orange-to-yellow, spindle-shaped insect; feeds in buds and flowers or on leaves (Figure 34) — IMMATURE THRIPS
Not as above — GO TO 14. -
Very small, scale-like insects on lower leaf surface; often associated with small, white, mothlike insects (Figure 35) — WHITEFLY NYMPHS
Active insects not associated with whiteflies, usually run or jump when disturbed — GO TO 15.
-
Hop or jump or run sideways (Figure 36) — LEAFHOPPER NYMPHS
Crawl or run away, but never sideways (Figure 37) — BUG NYMPHS
Stem Borers
- Head very dark (appears black); body slender, white, and legless (Figure 38) —FUNGUS GNAT MAGGOTS
Not as above; legs usually present — GO TO 2.
-
Cylindrical body with three pairs of legs near the head and five pairs of prolegs (Figure 39) — MOTH CATERPILLARS
Body flattened somewhat; usually three pairs of tiny legs near the head (in some cases, legs not present) (Figure 40) — BEETLE GRUBS
Root or Tuber Feeders
-
Legless — GO TO 2.
Three or more pairs of legs — GO TO 6. -
Body soft, slimy, dark-gray or black with a ridge down the back and a diamond shape near the center; tunnels through soil (Figure 41) — GREENHOUSE SLUG
Not a slug — GO TO 3. -
Dark head contrasting with light-colored body — GO TO 5.
Head not easily distinguishable from the rest of the body — GO TO 4. -
Fairly hard-bodied; yellow or white; slender and roughly cylindrical with pointed head and rounded abdomen (Figure 42) — MAGGOTS
Fairly soft-bodied, grub-like insect; usually in or near damaged bulbs or corms (Figure 43) — BULB FLY MAGGOTS -
Head very dark (almost black); body slender and white (Figure 44) — FUNGUS GNAT MAGGOTS
Head brown to black; body plump and usually curved or C-shaped; lacks legs (Figure 45) — WEEVIL GRUBS
-
Two pairs of legs on most segments; wormlike; hard-bodied, with three to many pairs of legs; curls up when disturbed (Figure 46) — MILLIPEDES
Not as above — GO TO 7. -
Three pairs of legs near the head and at least one pair of short, stumpy prolegs near the tip of the abdomen — GO TO 8.
Three pairs of legs and no short, stumpy prolegs on abdomen — GO TO 9. -
Three pairs of legs near the head and three to five pairs of prolegs on the abdomen (Figure 47) — MOTH CATERPILLARS
Sometimes three pairs of legs near the head and sometimes a pair of prolegs near the tip of the abdomen (Figure 48) — BEETLE LARVAE -
Front legs shovel-like for digging; dark brown insect covered with short, fine hairs (Figure 49) — MOLE CRICKET NYMPH
Front legs not shovel-like — GO TO 10. -
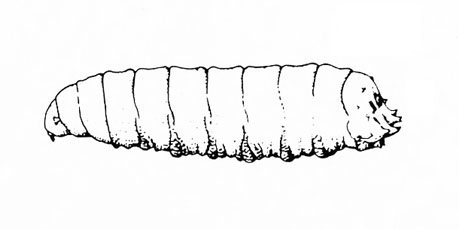
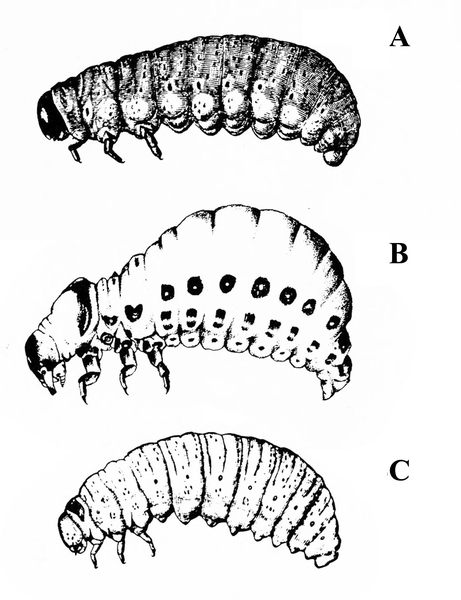
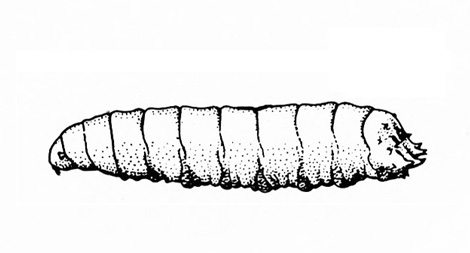
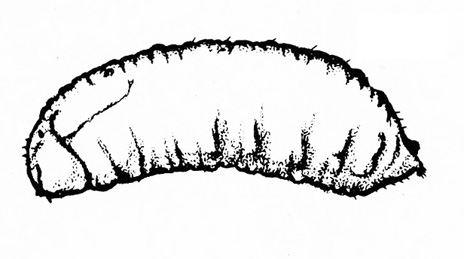
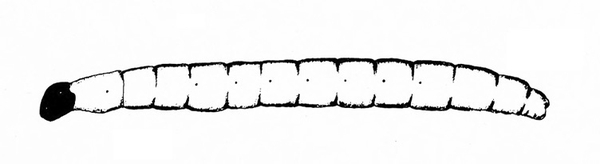
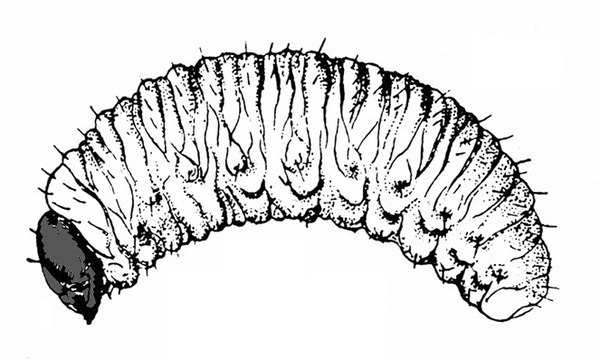
White or dirty-white grubs with dark head; wide variation in size; body slightly C-shaped and darker in color (Figure 50A–C) — BEETLE GRUBS
Very small jumping insect (usually 1/16 long, although rarely some grow to 1/8 inch long) with relatively short legs; sometimes with blunt antennae (Figure 51) — SPRINGTAIL NYMPH
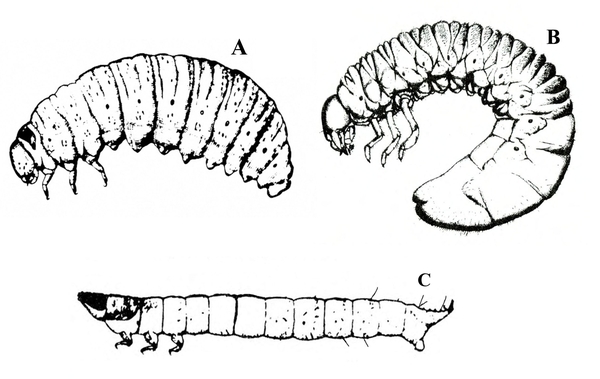
Figure 50A–C. Leaf beetle grubs (A) are often plump with downward-pointed heads. White grubs (B) are usually C-shaped and pale with brown to dark brown, downward-pointed heads. Wireworm grubs (C) are usually slender, brownish, and tough with wedge-shaped heads and acute tails.
Illustrations by USDA (A, C) and Ponglerd Kooaroon (B).
Publication date: May 29, 2024
AG-295
Recommendations for the use of agricultural chemicals are included in this publication as a convenience to the reader. The use of brand names and any mention or listing of commercial products or services in this publication does not imply endorsement by NC State University or N.C. A&T State University nor discrimination against similar products or services not mentioned. Individuals who use agricultural chemicals are responsible for ensuring that the intended use complies with current regulations and conforms to the product label. Be sure to obtain current information about usage regulations and examine a current product label before applying any chemical. For assistance, contact your local N.C. Cooperative Extension county center.
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.