Introduction
Biotechnology refers to the field of science where genetic material, living organisms, cells, and biological systems can be studied or used to create products and technologies. For instance, genetic engineering refers to a powerful set of tools within the field of biotechnology. By using genetic engineering in food, agricultural, and environmental contexts, scientists have been able to develop new food products, crop varieties, and approaches to potentially restore ecosystems, among other examples. Many of these applications aim to improve or enhance food production, quality, and environmental conditions.
At the same time, there have been significant discussions and public debates over the past few decades about the role of genetic engineering and its use in different fields. Today, scientists and regulatory officials continue to work together with other stakeholders from industry, non-governmental organizations (NGOs), and members of the public to understand and address these concerns. This work also aims to refine approaches to evaluate safety and ensure sufficient regulatory oversight of genetic engineering and its use in various products and within different contexts.
This publication outlines and describes core concepts related to genetic engineering and its use in food, agriculture, and the environment. This information may be particularly helpful for Extension agents, researchers, community members, government officials, and others who wish to better understand genetic engineering and the role it plays in our society.
This publication aims to discuss the following questions:
- What is genetic engineering?
- How is genetic engineering used in food and agriculture?
- How do I know which foods have been genetically engineered?
- Are genetically engineered foods safe to eat?
- What other social and ethical considerations have been discussed for genetically engineered foods and agricultural products?
- How is genetic engineering used for environmental purposes?
- What are the potential risks and ethical concerns of using genetic engineering for environmental purposes?
- How is genetic engineering regulated?
- How can I learn more?
This publication concludes with a list of resources for more information about genetic engineering as applied in food and agricultural systems and the environment.
What Is Genetic Engineering?
Genetic engineering refers to a set of techniques designed to change or alter the genetic makeup of an organism by introducing, removing, or modifying specific genes. An organism is a living being and includes all forms of life, such as bacteria, plants, animals, and fungi. All organisms possess genetic material (deoxyribonucleic acid, or DNA) containing information that controls an organism’s ability to function, develop, and reproduce, which is passed on to its offspring. Genes are the parts of the genetic material of an organism that most directly code for inherited traits that are passed on from one generation to the next. Typically, a gene contains a specific segment of DNA encoding a functional product, such as a protein, and can affect various traits and characteristics of organisms, such as hair and eye color in animals, as well as flower color and seed shape in plants, among others. Most traits are not controlled by only one gene; instead, they result from many genes acting in concert and responding to the environment.
Genetic engineering techniques can be used to introduce a new trait or characteristic to an organism or to enhance existing traits by directly altering its genetic material. An organism that is produced through genetic engineering is called a genetically engineered organism, also known more commonly as a genetically modified organism (GMO).
The term transgenesis refers to a genetic engineering process in which a sequence of DNA from one species of organism is introduced into a different species. Most commercially grown, genetically engineered crops have been developed using this approach. In some applications, a gene from a naturally occurring bacterium that codes for a protein that is toxic to some insect pests was moved into crops which in turn makes those crops toxic to the insects. Examples of this are Bt corn and Bt cotton, which contain a gene from Bacillus thuringiensis (Bt) that codes for a protein that controls caterpillars.
Gene editing is a relatively new genetic engineering method that can edit an organism’s genetic material and does not necessarily involve using another organism’s DNA. When no DNA from another organism is introduced, this is called a cisgenesis modification. Among other new techniques, the CRISPR system is a recently developed gene editing technique that acts like a pair of “molecular scissors” to cut an organism’s DNA in a precise location and either insert a new sequence of DNA, delete a piece of DNA, or substitute one piece of DNA for another piece (Figure 1). Genetic engineering, including CRISPR and other tools, has been used in a wide range of applications, including food, agriculture, environmental conservation, medicine, and industry.
How Is Genetic Engineering Used in Food and Agriculture?
Genetic engineering is used to enhance food and agricultural production by increasing yields, reducing pest and disease damage, improving food nutrition and quality, and contributing to sustainable agricultural practices. In plants, genetic engineering has enhanced crop characteristics, decreased insecticide use, improved tolerance to herbicides, and adapted plants to the impacts of climate change (such as drought and salinity resistance). For example, a type of corn called DroughtGard® has been genetically engineered to include a gene from the Bacillus subtilis bacterium to improve its tolerance to drought (Bayer, 2023; Nemali et al., 2014). There are also genetically engineered crops with improved health and nutritional qualities, such as rice engineered to have increased beta-carotene content (Golden Rice), soybean with increased omega-3 fatty acid content, and potatoes with lower acrylamide content (NASEM 2016 report).
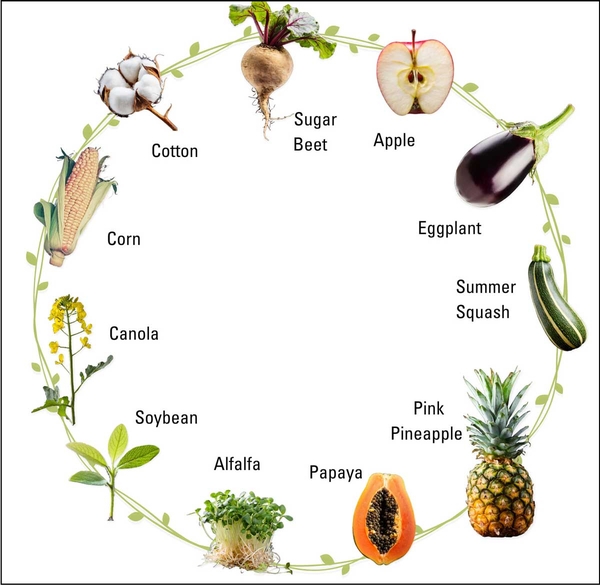
Today, there are several genetically modified crops available for purchase in the United States, including sugar beets, canola, corn, soybean, cotton, alfalfa, eggplant, potato, summer squash, papaya, and plum, as well as a few varieties of pineapple and apples (FDA 2022a; USDA n.d.a) (Figure 2). A genetically engineered purple tomato with high antioxidant properties has received USDA approval and has hit the market recently (Norfolk Healthy Produce 2023). Other products that are expected to reach the market in the coming years include gene-edited, pitless or seedless cherries and mangoes (Pairwise 2023) and disease-resistant bananas (FoodIndredientsFirst 2023).
Most genetically modified crops (including corn, soybean, and alfalfa) are used to feed animals, such as cows, poultry, and fish. Today, a consumer may only encounter a few, if any, of the aforementioned genetically engineered fruits or vegetables during a trip to the fresh produce aisle of a US supermarket. This is because items in a supermarket that are most likely to contain engineered ingredients are processed foods with flour, sugars, and oils from engineered corn and soybeans. This may change in the next decade if engineered fresh produce finds a bigger market niche.
There are some genetically engineered animals on the market and others in development. For example, AquAdvantage Salmon has been genetically modified to grow at a faster rate than its conventional counterpart by using genes from Chinook salmon and an ocean pout (FDA 2023b). The Food and Drug Administration (FDA) has approved AquAdvantage for sale in the United States. Further, a type of cattle has been recently produced using gene editing to have a slick hair coat, which can improve its ability to withstand higher temperatures (CRISPR Beef Cattle Get FDA Green Light 2022). It has been reviewed and approved by the FDA and is expected to reach the US market soon (FDA 2022a). Other recent developments include genetically engineered microbes for use in various applications, including in livestock or directly in agricultural soils, although most of these are still in the research and development phases.
How Do I Know Which Foods Have Been Genetically Engineered?
There are a few different approaches to inform consumers about whether a food or food product has been genetically engineered or contains genetically engineered ingredients.
Food labeling and disclosure laws are both options, and they tend to vary by country. Food labeling involves the presentation of information on food packaging and includes detailed information such as product name, ingredients, nutritional content, allergens, serving size, and other information. Food disclosures are broader than labeling and include not only information on a label but also information provided through other means such as online platforms or nutritional information available in restaurants.
In the United States, the federal government passed the National Bioengineered Food Disclosure Standard (NBFDS) in 2016. Since January 1, 2022, this law requires foods that contain genetically engineered ingredients to include transparent information for consumers about the food they purchase and whether they contain genetically engineered ingredients. This law uses the term “bioengineered” instead of “genetic engineering,” although these terms are essentially the same. For any new bioengineered foods (such as Bt sugarcane) that are added to an updated list of bioengineered foods, food manufacturers have until July 1, 2025, to comply with this law (USDA n.d.b). The United States Department of Agriculture (USDA) is the federal agency responsible for implementing and overseeing the food disclosure standard in the United States In addition to food labeling requirements, the FDA and USDA publish lists of foods that have been genetically engineered on their websites (FDA 2022a; USDA n.d.a).
There are three general approaches for disclosing a food containing genetically engineered or bioengineered ingredients according to the NBFDS: (1) the list of ingredients on the food package can contain information indicating that the food contains bioengineered ingredients (Figure 3A), (2) the manufacturers may use a symbol or a QR code that provides a link to a web page with more information (Figures 3B and 3C), and (3) the food manufacturers can place a phone number on the food package that consumers can call or text to receive information about the bioengineered content. While food companies may use other terms such as “genetically modified organism,” “GMO,” and “genetic engineering” on food labels, they are required to use “bioengineered food” or “contains bioengineered ingredient(s)” according to this food disclosure law. In addition, the US food disclosure law does not require that the manufacturer or food producer specify which ingredients within the food are bioengineered.
If a food is labeled as containing bioengineered ingredients, this means that it contains detectable levels of genetic material that has been modified through genetic engineering techniques that could not be created through conventional breeding processes. This also extends to gene-edited food ingredients. In these cases, USDA investigates each bioengineered food on a case-by-case basis to determine if it contains “modified DNA” in the final product. If so, then it requires disclosure according to the NBFDS rule. Note that animal products derived from animals that are fed genetically engineered crops but not genetically engineered themselves, such as meat, poultry, and eggs, do not need to be labeled or disclosed. In addition, food that is sold at very small food manufacturers, restaurants, food trucks, within trains and airplanes, or present in very small portions of food ingredients (that is, less than 5%) are exempt from this disclosure law (USDA n.d.c; National Bioengineered Food Disclosure Standard 2018).

Figure 3. Foods that are genetically engineered (also called bioengineered) must be labeled after July 2025, according to the US NBFDS. The options for labeling foods include (A) listing bioengineered ingredients, (B) using a bioengineered symbol, or (C) using a QR code that provides an additional link on food packaging.
Are Genetically Engineered Foods Safe to Eat?
Significant research has been conducted over the past several decades on the safety of genetically engineered foods and food ingredients for human consumption. Risk and safety studies focus on identifying new or different hazards resulting from engineering or modifying an organism's genetic material through genetic engineering techniques. This research has been conducted by large scientific bodies of experts, including the World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO), US National Academies of Sciences, and European Food Safety Authority (EFSA), as well as many other organizations and individual investigators.
Across these investigations, there has not been compelling evidence or data to directly link the consumption of genetically engineered crops with adverse health effects. These studies have relied on both short-term and long-term toxicity investigations using animals, studies of disease and chronic conditions in people (such as cancer, obesity, diabetes, and gastrointestinal issues), as well as investigations into their nutritional value and allergenicity (for instance, gluten allergenicity). Further, these research investigations have indicated that currently marketed, genetically engineered foods are expected to be just as safe and nutritious to consume as their non-genetically engineered counterparts.
Similarly, studies that have focused on the safety of genetically engineered foods for consumption by livestock animals also show that they are as safe as their non-genetically engineered counterparts (NASEM 2016). In addition, the genetically engineered components of the animal feed do not transfer to the animal or to any animal products they produce (such as milk, eggs, and meat).
What Other Social or Ethical Considerations Have Been Discussed for Genetically Engineered Foods or Agricultural Products?
Over the past few decades, significant social and ethical concerns have been raised by the development and use of genetic engineering, particularly in food and agriculture products. One set of these concerns is related to potential unintended impacts on the environment, such as the potential for modified or engineered genes to spread into wild populations, as well as impacts on biological diversity in ecological systems. The US Environmental Protection Agency (EPA), USDA, and FDA are responsible for investigating these potential impacts through risk assessment processes in their respective jurisdictions. A second set of concerns relates to issues of transparency and consumer awareness of genetically engineered foods or food ingredients in the food supply and the ability of consumers to make informed decisions. For these reasons, the United States has introduced the National Bioengineered Food Disclosure standard that labels foods containing bioengineered ingredients (see previous section). Concerns about transparency have also centered on the regulation and approval of genetically engineered products as updates to rules in various agencies have increased the number of genetically engineered products that are exempt from regulation, and much of the information leading to approval is not always fully disclosed. In addition, the developer is primarily responsible for transparency, which is voluntary.
There have also been ethical concerns regarding the use of genetic engineering in animals, primarily regarding their treatment and well-being. For these reasons, among others, several groups have not accepted the use of genetic engineering in food and agriculture products.
How Is Genetic Engineering Used for Environmental Purposes?
In addition to applications in food and agriculture, genetic engineering has been proposed to help solve environmental challenges. Genetic engineering may help conserve and restore biological diversity, develop plants and animals that are more resilient to climate change, create biofuels and bioplastics, reduce greenhouse gas emissions, manage invasive species, and create trees that can absorb more carbon dioxide from the atmosphere, among other examples.
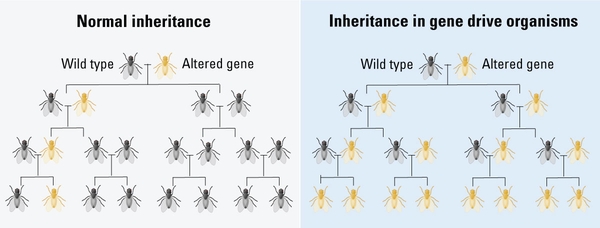
Gene drives are a tool of genetic engineering that can help spread certain genetic traits in a population and may be used for environmental conservation purposes. Rather than relying on the normal rules of genetic inheritance, gene drives use another type of genetic “super” inheritance that increases the probability of inheriting a gene, which in turn increases the prevalence of specific genes across a population. For example, in normal inheritance, a gene has an equal probability (50-50) of being passed on from parent to their offspring. Gene drives can increase that likelihood to greater than 95% (Figure 4).
Some biologists and conservationists are interested in using gene drives for environmental conservation purposes, including the management of invasive species and biodiversity conservation. For example, researchers and conservation groups (such as Island Conservation) are developing gene drive systems in mice to help eradicate invasive mice populations that threaten biodiversity and native ecosystems on islands (Godwin et al. 2019; Hay and Guo 2022). In these cases, invasive mice and other rodents threaten endemic species on islands, resulting in a loss of biodiversity. This is particularly concerning because many islands contain species that are found nowhere else on Earth (Barnhill-Dilling et al. 2019). Traditional control measures, such as rodenticides, have not proven effective enough and cannot be used on a large percentage of islands where invasive mice are impacting local ecosystems and human communities. Rodenticides are broad-spectrum toxicants, which means that they often have unintended effects on other species, including negative impacts on the very species conservation groups are trying to protect (Barnhill-Dilling and Delborne 2021). Therefore, researchers are exploring the use of gene drives for more effective, long-term solutions to eradicating invasive mice populations on islands and restoring ecological biodiversity. Currently, gene drive mice are being developed in laboratories, and meanwhile, other stakeholders, such as the international consortium Genetic Biocontrol of Invasive Rodents (GBIRd) are exploring potential ecological impacts and public perceptions of using these gene drives.
Other examples of using genetic engineering to help solve environmental challenges include genetically engineered corals that are heat-resistant in response to warming marine environments (Hobman et al. 2022; Cornwall 2019), CRISPR-mediated gene editing to create more efficient biofuels production (Lakhawat et al. 2022), and genetically engineered crops for better utilization of nutrients such as nitrogen and phosphorus (Lebedev et al. 2021; Gaxiola et al. 2011), among other examples.
What Are the Potential Risks or Ethical Concerns of Using Genetic Engineering for Environmental Purposes?
Similar to concerns raised about the use of genetic engineering in other applications, its use to help solve environmental challenges has also raised a number of health, environmental, social, ethical, and regulatory concerns. For example, concerns have been raised about genetically engineered trees regarding the potential of engineered or modified genes to escape to sexually compatible trees and unintended effects on other environmental organisms. There are also issues raised about the proprietary aspects of genetically engineered trees, including ownership, control, and regulation (Pinchot 2014).
Some broader concerns about the use of genetic engineering are focused on the possible increased use of agrochemicals (like fertilizers and pesticides), the use of environmental resources such as water and land use, and the impacts that genetic engineering may have on climate change with a possible increase in greenhouse gas emissions (Kuzma 2023). At the same time, and as mentioned above, many proponents of genetic engineering suggest that there may be environmental benefits to many of these agricultural and environmental applications, including using fewer agrochemicals and potentially decreasing greenhouse gas emissions (Brookes 2022).
In addition, other concerns have been raised related to the development and release of gene drive organisms. These include potential unintended effects on other non-target species, the ability for engineered or modified genes to transfer to other sexually compatible species, unintended environmental effects, and ethical and regulatory implications (Kormos et al. 2022; Hartley et al. 2022). Concerns have also been raised about how effective the technology is and how to ensure it can be stopped if needed, along with many ethical concerns focused on island-based nations being testing sites for the technology.
How Is Genetic Engineering Regulated by Government Agencies?
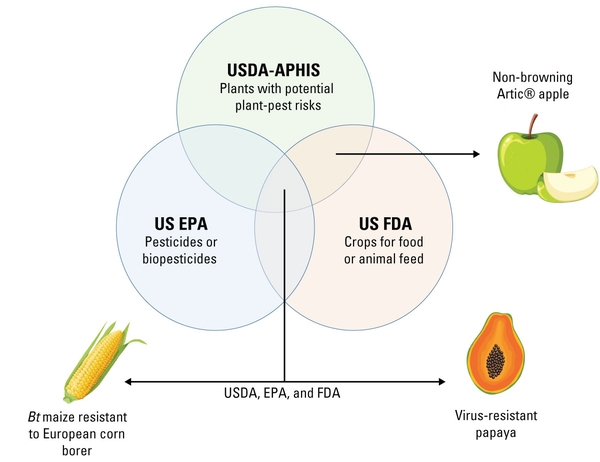
Genetic engineering regulation varies by country. In the United States, products developed using genetic engineering are regulated by three different government agencies under the Coordinated Framework for Regulation of Biotechnology, established in 1986 (US Office of Science and Technology Policy 2017). These agencies include the US EPA, FDA, and USDA. Each agency is tasked with specific responsibilities related to the safety and regulatory approval of products that have been genetically engineered or contain genetically engineered components under their jurisdiction (Figure 5). The FDA is tasked with regulating human and animal food and feed, ensuring food and feed are safe for consumption. The US EPA regulates pesticides, including substances known as plant-incorporated protectants (PIPs) that may be present in some genetically engineered crops, ensuring that these products do not pose unintended or unreasonable risks to humans, animals, or the environment. The USDA (specifically the Animal and Plant Health Inspection Service, or APHIS) safeguards plant and animal health, including protecting agriculture and agriculturally important resources by ensuring that genetically engineered crops do not have harmful side effects on agricultural systems or the environment. Together, USDA, FDA, and EPA are responsible for ensuring genetically engineered organisms do not present risks to human health or the environment. These agencies also continuously update their oversight and guidance as the technologies and products change. They also consider input from the White House, such as the recent Executive Order on Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy, which includes genetic engineering and calls for changes in its oversight.
The regulation of genetically engineered organisms that are not related to food or agricultural products, such as trees or gene drive mice, also falls under the jurisdiction of these agencies, depending on existing laws and statutes as part of the Coordinated Framework for the Regulation of Biotechnology.
Summary
Genetic engineering is a powerful set of biotechnology tools that allow for the deliberate modification of an organism's genetic makeup by introducing, removing, or altering specific genes to introduce new traits or enhance existing ones. New gene editing techniques, such as CRISPR, have been developed in recent years.
Today, there are more than a dozen food and agricultural products on the US market that have been directly genetically engineered. In addition, genetic engineering has been proposed to help solve different environmental challenges, including restoring the environment, conserving biodiversity, and adapting to the effects of climate change.
While genetic engineering has potential benefits, concerns continue to be raised about its safety, oversight, and regulation. Today, three regulatory agencies in the United States, the USDA, FDA, and EPA, are responsible for regulating genetic engineering through the Coordinated Framework for Regulation of Biotechnology.
How Can I Learn More?
The following resources may be particularly helpful and informative for learning more about genetic engineering and its applications in food, agriculture, and the environment. Additional references are included in the subsequent section.
- National Academies of Sciences, Engineering, and Medicine (NASEM). 2022a. “Are All Crops That We Eat Genetically Improved?”
- NASEM. 2022b. “Foods Made with GMOs Do Not Pose Special Health Risks.”
- The United Website for Biotechnology Regulation. 2024. “About the Coordinated Framework.” US Department of Agriculture (USDA), US Food and Drug Administration (FDA), and Environmental Protection Agency (EPA).
- US Department of Agriculture (USDA). 2024. “Biotechnology Frequently Asked Questions (FAQs).”
- US Environmental Protection Agency (EPA). 2023. “Genetically Modified Organisms.”
- US Food and Drug Administration (FDA). 2023. “Agricultural Biotechnology: Feed Your Mind.”
References
Barnhill-Dilling, S. K., Serr, M., Blondel, D. V., & Godwin, J. 2019. “Sustainability as a Framework for Considering Gene Drive Mice for Invasive Rodent Eradication.” Sustainability 11, no. 5: 1334. ↲
Barnhill‐Dilling, S. K., & Delborne, J. A. 2021. “Whose Intentions? What Consequences? Interrogating ‘Intended Consequences’ for Conservation with Environmental Biotechnology.” Conservation Science and Practice 3, no. 4: e406. ↲
Bayer. 2023. “DroughtGard® Hybrids.” Drought Protection, Bayer Traits, Crop Science US.” Accessed January 26, 2024. ↲
Brookes, G. 2022. “Genetically Modified (GM) Crop Use 1996–2020: Environmental Impacts Associated with Pesticide Use Change.” GM Crops Food 13, no. 1: 262–289. ↲
Cornwall. W. 2019. “Researchers embrace a radical idea: Engineering coral to cope with climate change.” Accessed January 26, 2024. ↲
CRISPR Beef Cattle Get FDA Green Light. 2022. Nature Biotechnology 40, no 4: Article 4. ↲
FoodIngredientsFirst. 2023. “Gene-Editing Tech Could Save Cavendish Bananas from Deadly Fungus Threatening Extinction.” Accessed January 26, 2024. ↲
FDA. 2020. “FDA Approves First-of-its-Kind Intentional Genomic Alteration in Line of Domestic Pigs for Both Human Food, Potential Therapeutic Uses.” Press Announcements, FDA. ↲
FDA. 2022a. “FDA Makes Low-Risk Determination for Marketing of Products from Genome-Edited Beef Cattle After Safety Review.” Press Announcements, FDA. ↲
FDA. 2023b. “AquAdvantage Salmon Fact Sheet.” AquAdvantage Salmon, FDA. ↲
Gaxiola, R. A., Edwards, M., & Elser, J. J. 2011. “A Transgenic Approach to Enhance Phosphorus Use Efficiency in Crops as Part of a Comprehensive Strategy for Sustainable Agriculture.” Chemosphere 84, no. 6: 840–845. ↲
Godwin, J., Serr, M., Barnhill-Dilling, S. K., Blondel, D. V., Brown, P. R., Campbell, K., Delborne, J., Lloyd, A. L., Oh, K. P., Prowse, T. A. A., Saah, R., & Thomas, P. 2019. “Rodent Gene Drives for Conservation: Opportunities and Data Needs.” Proceedings. Biological Sciences 286, no. 1914: 20191606. ↲
Grieger, K., Wiener, J. Kuzma, J. 2024. “Improving Risk Governance Strategies via Two-way Learning: A Comparative Analysis of Solar Geoengineering and Gene Drives.” Environment Systems and Decisions. ↲
Hartley, S., Taitingfong, R., & Fidelman, P. 2022. “The Principles Driving Gene Drives for Conservation.” Environmental Science & Policy 135 (September 2022): 36–45. ↲
Hay, B. A., & Guo, M. 2022. “Gene Drive-Mediated Population Elimination for Biodiversity Conservation. When You Come to a Fork in the Road, Take It.” Proceedings of the National Academy of Sciences 119, no. 51: e2218020119. ↲
Hobman, E. V., Mankad, A., Carter, L., & Ruttley, C. 2022. “Genetically Engineered Heat-Resistant Coral: An Initial Analysis of Public Opinion." PLoS ONE 17, no. 1: e0252739. ↲
Hoofprint Biome (website). 2023. Accessed January 26, 2024. ↲
Kormos, A., Lanzaro, G. C., Bier, E., Santos, V., Nazaré, L., Pinto, J., Aguiar dos Santos, A., & James, A. A. 2022. “Ethical Considerations for Gene Drive: Challenges of Balancing Inclusion, Power and Perspectives.” Frontiers in Bioengineering and Biotechnology 10. ↲
Kuzma, J., Grieger, K., Cimadori, I., Cummings, C. L., Loschin, N., & Wei, W. 2023. “Parameters, Practices, and Preferences for Regulatory Review of Emerging Biotechnology Products in Food and Agriculture." Frontiers in Bioengineering and Biotechnology 11: 1256388. ↲
Lakhawat, S. S., Malik, N., Kumar, V., Kumar, S., & Sharma, P. K. 2022. “Implications of CRISPR-Cas9 in Developing Next Generation Biofuel: A Mini-Review.” Current Protein & Peptide Science 23, no. 9: 574–584. ↲
Lebedev, V. G., Popova, A. A., & Shestibratov, K. A. 2021. “Genetic Engineering and Genome Editing for Improving Nitrogen Use Efficiency in Plants.” Cells 10, no. 12: 3303. ↲
National Bioengineered Food Disclosure Standard. 2018. Federal Register. ↲
NASEM. 2016. Genetically Engineered Crops: Experiences and Prospects | The National Academies Press. Accessed January 26, 2024. ↲
Nemali, K.S., Bonin, C., Dohleman, F.G., Stephens, M., Reeves, W.R., Nelson, D.E., Castiglioni, P., Whitsel, J.E., Sammons, B., Silady, R.A., Anstrom, D., Sharp, R., Patharkar, O.R., Clay, D., Coffin, M., Nementh, M., Leibman, M.E., Luethy, M., & Lawson, M. 2014 . “Physiological responses related to increased grain yield under drought in the first biotechnology-derived drought-tolerant maize.” Plant, Cell & Environment 38, no. 9:1866-1880. ↲
Norfolk Healthy Produce (website). 2023. Accessed January 26, 2024. ↲
Pairwise. 2023. “Conscious Foods.” Accessed January 26, 2024. ↲
Pinchot, L. 2014. “American Chestnut: A Test Case for Genetic Engineering?” US Forest Service. Wisdom, (spring-summer 2014): 8–15. ↲
USDA. n.d.a. “List of Bioengineered Foods.” Agricultural Marketing Service. Accessed January 26, 2024. ↲
USDA. n.d.b. “Industry Fact Sheet – National Bioengineered Food Disclosure Standard.” Agricultural Marketing Service. Accessed January 26, 2024. ↲
USDA. n.d.c. “BE Disclosure.” Agricultural Marketing Service. Accessed January 26, 2024. ↲
US Office of Science and Technology Policy. 2017. Modernizing the Regulatory System for Biotechnology Products: Final Version of the 2017 Update to the Coordinated Framework for the Regulation of Biotechnology. ↲
Acknowledgment
This work is supported by the A1642 Social Implications of Food and Agriculture Technologies program, project award no. 2022-67023-36730, from the U.S. Department of Agriculture’s National Institute of Food and Agriculture. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and should not be construed to represent any official USDA or U.S. Government determination or policy.
Publication date: July 23, 2024
AG-967
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.