Introduction
Corn is the 2nd crop in North Carolina, planted over approximately 830,000 acres of land and valued at over $700 million annually (USDA, 2022). The crop can be used as livestock feed and can be processed into fuel, oil, beverages, starch, and sweeteners. Corn can be grown in the field and in gardens at home; however, corn is susceptible to plant-parasitic nematodes. These are soilborne, microscopic roundworms that infest and feed on plant roots. There are numerous genera (that is, types or groups), of plant-parasitic nematodes, yet one group that is particularly impactful to corn production is root-knot nematodes.
However, not all nematodes found in the soil of corn are plant-parasitic, and therefore, not all nematodes found in soil are harmful. Free-living nematodes may aid in the growth of corn by breaking down nutrients and preying on other pathogens and pests.
Pathogen
Meloidogyne, and there are four “major” root-knot nematode species, namely Meloidogyne incognita (Southern root-knot nematode), Meloidogyne javanica (Javanese root-knot nematode), Meloidogyne hapla (Northern root-knot nematode), and Meloidogyne arenaria (peanut root-knot nematode). Of these, the Southern root-knot, Javanese root-knot, and peanut root-knot are able to infect and feed on corn. Corn is a weak or poor host to the Northern root-knot nematode. Recently, Meloidogyne enterolobii (guava root-knot nematode) has been identified in North Carolina. Corn is a poor host to the guava root-knot nematode.
This disease factsheet will focus on the Southern root-knot nematode (M. incognita), because is the most widespread root-knot nematode species across North Carolina. This species can be found in tropic to subtropical regions such as southern U.S.A., China, central Europe, and Brazil.
Host Range
The Southern root-knot nematode (Meloidogyne incognita) has an extensive host range including vegetables, herbs, field crops, fruits, trees, ornamentals, and weeds (Table1). Host plants are crops upon which the nematode is able to feed and complete its life cycle.
| Crop Type | Host Crop Examples | Non-Host Crop Examples |
|---|---|---|
| Vegetables and Herbs | Many vegetables, including tomatoes, sweetpotato, pepper, eggplant, okra, basil, bean, cabbage, carrot, lettuce, potato | |
| Field Crops | Corn, cotton, soybean, sorghum, tobacco, wheat, rye, oats, barley, alfalfa | Peanut, sesame, sunn hemp |
| Fruit and Trees | Many fruits, including watermelon, cantaloupe, cucumber, peach, plantain | Strawberry1 |
| Weeds | Many weeds, including nutsedge, morning glory, crabgrass, lambsquarter, sicklepod, pigweed | Jimsonweed, Johnsonweed, ragweed |
| Ornamentals | Many annual and perennial ornamental plants, including pansy, verbena, celosia | Marigold |
1 The host status of strawberry to Southern root-knot nematode (M. incognita) is somewhat inconclusive, but some varieties of strawberry are reported as resistant. ↲
Life Cycle and Favorable Conditions for Disease
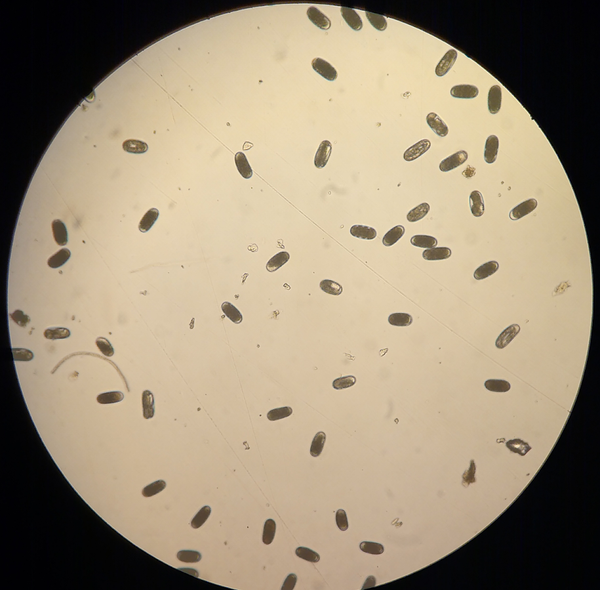
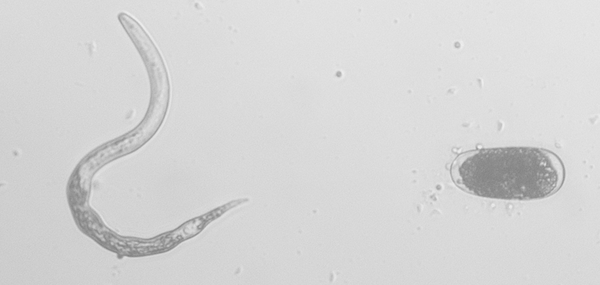
The Southern root-knot nematode has a life cycle that is similar to other root-knot nematode species. To begin, root-knot nematodes hatch from their tic-tac-shaped eggs (Fig. 1) in warm, moist soil. Once hatched, the nematodes are referred to infectious second-stage juveniles (Fig. 2) and these juveniles will search for and penetrate actively growing plant roots near the root tips. Here, they establish feeding sites called “giant cells”. The nematodes will then molt three times to become adults, and are fully enclosed by the root tissues. At the adult stage, root-knot nematode females swell in size. This swelling, along with the giant cells, creates the characteristic root galls or knots for which the group is named (Fig 3). Root-knot nematode in corn tends to produce less conspicuous and visible galls than other crops, such as soybean, cotton, or tobacco.
Adult female Southern root-knot nematodes typically lay approximately 150-1500 eggs each, deposited in a gelatinous-like egg mass outside of the root. In optimal conditions, it typically takes 2-5 weeks to complete their life cycle. Although root-knot nematodes can infest crops in a variety of soil types, root-knot nematodes thrive in sandy soils in tropical to subtropical regions.
Signs and Symptoms
Detecting root-knot nematodes in corn may be difficult because symptoms observed above ground often resemble nutrient deficiencies and/or drought stress. Above-ground symptoms of Southern root-knot nematode in corn may include wilting, chlorosis and yellowing (Fig. 4), stunted growth and reduced yields (Fig. 5), or even plant death at high densities. Above-ground symptoms are frequently observed in distinct patches or “disease foci” across the field, corresponding to where the nematode population is moderate to high.
Below ground, root-knot nematode infestations can often be identified in the field by the presence of root galls, knots, or bumps on the root system (Fig. 6). However, Southern root-knot nematode on corn produces less conspicuous or visible galls than on other crops such as soybean, cotton, or tobacco.
Diagnosis
If a corn crop is suspected to be impacted by southern root-knot nematode, a few plants should be dug up, taking care to preserve the integrity of the root system. Visually inspect root, looking for the presence of root galls (Fig. 6), which indicate infection of the corn plant by root-knot nematodes (note that these root galls are likely to be small. Use of a hand lens or magnifying glass may help to look for them). Although the presence of root galls is sufficient to diagnose root-knot nematodes in corn, root galling symptoms alone will not indicate the level of nematode pressure (nematode population counts) in the field, nor indicate what specific species of root-knot nematode is present. Diagnosis can be confirmed through assessment of soil and root samples for the presence of root-knot nematodes by a plant disease diagnostic laboratory or nematode assay lab. Soil samples for nematode assay services may be submitted to the North Carolina Department of Agriculture & Consumer Services (NCDA&CS), Agronomic Services, Nematode Assay Laboratory, 4300 Reedy Creek Road, Raleigh, NC 27607.
Nematode populations vary throughout a field, so multiple areas of the field should be sampled to provide an accurate evaluation of the entire field. Using a soil probe or hand trowel, collect 20 to 30 soil cores from a 5-acre block, at a depth of 6-8 inches, along either a grid-like or a zig-zag pattern across the field. Combine the soil cores in a clean plastic bucket and remove a smaller subsample from this larger sample to submit to the Nematode Assay Laboratory. Detailed sampling instructions and sample submission forms can be found at the NCDA&CS Nematode Assay Laboratory sampling guidelines.
The standard nematode soil assay cannot differentiate between specific root-knot nematode species, due to the highly similar appearance of these nematode species under the microscope. Root-knot nematode species identification can be obtained through a molecular assay at a diagnostic lab. This molecular diagnosis must be requested when submitting a sample and usually requires an additional fee. When requesting a molecular assay, it is helpful to include roots with distinct root galls at the time of sample submission as well as an accurate crop history, information about fertility, herbicides, and cultural practices to aid in diagnosis.
Management
Managing Southern root-knot nematode can be difficult to its broad host range (Table 1) and the lack of corn varieties with known resistance to Southern root-knot nematode. Routine soil sampling for nematode analysis is important to understand if a field has root-knot nematode and, if so, to monitor population counts. Knowledge of specific root-knot nematode species in the field is helpful for determining if there is a non-host crop that can be integrated into the cropping sequence. However, this species-level information frequently requires a DNA-based test. Southern root-knot nematode can also infect, reproduce, and survive on common weeds. Therefore good weed management will eliminate a potential food source for these nematodes.
Integrated pest management (IPM) is a “toolbox approach” used to reduce disease pressure and populations of pest organisms by using several disease management tactics in conjunction with each other. Rotating the use of and/or combining management tactics lessens reliance on a single tactic, minimizes costs, and minimizes risks of developing resistance to a single pesticide. The primary management strategies in the IPM toolbox are outlined below.
However, an important first step to implementing an IPM approach is to determine when to make a nematicide treatment or use another IPM tactic. Using a pathogen population threshold can aid in determining when to use management strategies and also reduces costs by avoiding treatments when populations are below economically impactful levels. Reviewing your nematode assay report to evaluate the population density of each nematode and risk level to corn.
Cultural
The cultural management strategy is modifying the environment or certain production practices to reduce the pest population. Examples of tactics within this strategy include:
- Sanitizing equipment and tools by removing infested soil stuck to equipment with a solution of 10% household bleach and rinsing with clean water before drying.
- Rotating to non-host crops of M. incognita, such as peanuts, which may reduce M. incognita populations by withholding a suitable host.
- Using non-host cover crops such as sunn hemp or marigold to cover the ground may improve soil fertility, soil structure, reduce soil erosion, and suppress weeds and other plant pathogens (Gill et al. 2023).
- Using resistant crop where available; certain varieties of tomato, soybean, cotton, and tobacco possess host genetic resistance to certain races of Southern root-knot nematode. However, no host resistance is known in commercial corn varieties to Southern or other root-knot nematode species.
Mechanical
Mechanical management tactics use physical means to block or destroy nematode pathogens. Examples of tactics within this strategy include:
- Disking or working the field several times before planting can reduce nematode numbers through abrasive action of soil particle movement on the nematode bodies.
- For when smaller areas need to be treated with non-chemical means, soil solarization may be viable. In this technique, clear plastic sheets are laid and sealed over the soil to raise the temperature of the soil using a greenhouse effect, which effectively kills disease-causing organisms in the soil. More information about soil solarization can be found in the University of California’s handbook.
Biological and Chemical
Biological and chemical management tactics use nematicidal compounds to reduce populations of the nematode. Nematicides can be used to manage Southern root-knot nematode in corn, where economically viable. Pre-plant soil fumigation, at-plant nematicides, or the use of nematicidal seed treatment can reduce Southern root-knot nematode population levels to below yield-impacting levels. Chemical and biological nematicide products are summarized in Table 2 and additional information may be found in the North Carolina Agricultural Chemicals Manual. While using chemicals to manage pathogens and pests is effective, there may be unintended effects such as phytotoxicity to the crop or residual toxicity to the environment. It is imperative to follow the label to minimize these risks.
| Material and Formulation | Trade Name | Application Rate per Acre (36 inch rows) | Notes |
|---|---|---|---|
| Terbufos
|
Counter 20G
|
5.0 lb | Apply in-furrow. Do not exceed 6.5 lbs per acre of Counter 20G. |
| Fluopyram 17.4% + Prothioconazole 17.4%
|
Propulse
|
8.0 fl oz | In-furrow spray during planting on or below seed. Tank mixes with Propulse and some fertilizers has been problematic. A “jar test” before tank mixing to test compatibility is recommended. See label for additional instructions. |
| Abamectin
|
Avicta
|
0.15 mg per seed | Seed treatment |
| Clothianidin 40.30% + Bacillus firmus I-1582 8.10%
|
Poncho/Votivo
|
2.7 fl oz per 80,000 seed | Seed treatment |
| Bacillus amyloiquefaciens strain PTA-4838 16.5%
|
Aveo EZ
|
0.1 fl oz per 80,000 seed | Seed treatment |
| Heat-killed Burkholderia spp. Strain A396 94.46%
|
BioST
|
8 fl oz per 100 lbs seed | Seed treatment |
Additional Resources
The NCDA&CS Nematode Assay Lab provides soil detection and diagnostics
The NC State University Plant Disease and Insect Clinic provides diagnostic and control recommendations
The NC State Extension Plant Pathology portal provides information on crop disease management
The North Carolina Agricultural Chemicals Manual provides an up-to-date list of chemicals available for control of nematodes and other diseases and pests
Acknowledgements
This factsheet was prepared by the NC State University Plant Nematology Lab in 2023. We thank the Corn Grower Association of North Carolina for providing support to prepare this factsheet.
References
- USDA National Agricultural Statistics Service. 2022. 2022 State Agriculture Overview, North Carolina.
- H. K. Gill, Z. J. Grabau, and R. McSorley. 2023. Cover Crops for Managing Root-Knot Nematodes.
Publication date: Sept. 1, 2023
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.
NC Cooperative Extension prohíbe la discriminación por raza, color, nacionalidad, edad, sexo (incluyendo el embarazo), discapacidad, religión, orientación sexual, identidad de género, información genética, afiliación política, y estatus de veteran.
The use of brand names in this publication does not imply endorsement by NC State University or N.C. A&T State University of the products or services named nor discrimination against similar products or services not mentioned.
Recommendations for the use of agricultural chemicals are included in this publication as a convenience to the reader. The use of brand names and any mention or listing of commercial products or services in this publication does not imply endorsement by NC State University or N.C. A&T State University nor discrimination against similar products or services not mentioned. Individuals who use agricultural chemicals are responsible for ensuring that the intended use complies with current regulations and conforms to the product label. Be sure to obtain current information about usage regulations and examine a current product label before applying any chemical. For assistance, contact your local N.C. Cooperative Extension county center.
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.