Production Management
Key management practices for organic wheat and small grain production include the following:
- Implementing crop rotation
- Burying crop residue with tillage, if possible
- Choosing varieties with resistance to disease and insect pests
- Planting on time (not too early, not too late) in a well-prepared seedbed
- Using correct seeding rates, drill calibration, and drill operation
- Avoiding excessively high nitrogen levels (but working towards good soil fertility)
- Harvesting on time and not letting mature grain stand in the field
Variety Selection
It is nearly impossible to pick a single best variety. Consequently, producers should plant at least two varieties every season to reduce their risks and maximize the potential for a high-yielding crop. The following are general guidelines for selecting varieties for organic wheat production:
- The two disease and pest problems with the greatest potential to completely devastate a small-grain crop in any part of North Carolina are Fusarium head blight (scab) and Hessian fly. Only choose varieties that are rated as moderately resistant (MR) to scab. Avoid varieties rated “poor” for Hessian fly, especially if planting will be early or even on time.
- To avoid spring freeze injury, avoid early-heading varieties in favor of medium- and late-heading varieties.
- If wheat is being produced for the baking industry, it is a good idea to check variety selection with the end user.
Planting Date
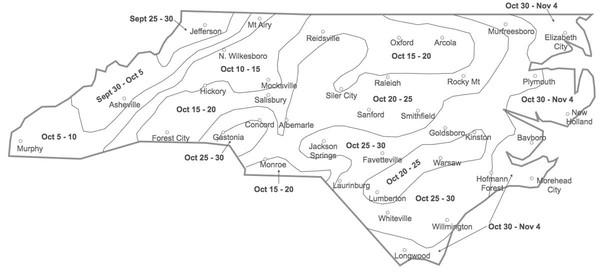
Not too early and not too late! Planting too early puts the crop at severe risk for powdery mildew, Hessian fly, aphids, and barley yellow dwarf virus. Planting too late will reduce yields, increase the risk of having a winter annual weed problem, and result in thin stands that will be more vulnerable to cereal leaf beetle feeding. For the optimum planting times for your region, see “Small Grain Planting Dates” in the Small Grain Production Guide (AG-850, revised).
Rotation and Field Selection
Planting wheat into old wheat stubble is not recommended. Several major small-grain diseases (Septoria nodorum blotch, tan spot, and scab) are transmitted by old wheat stubble. Short small grain rotations also put the crop at high risk to soilborne diseases like take-all. Planting wheat two years in a row is not recommended for conventional or organic systems.
Hessian fly pupae oversummer in wheat residues, so planting into or near old wheat stubble risks Hessian fly infestation in a new wheat crop. The best way to avoid a Hessian fly problem is to plant at least one field (or ¼ mile) away from last year’s wheat stubble and avoid planting near an early-planted wheat cover crop.
Fields with a history of Italian ryegrass or wild garlic should be avoided because there are no good organic methods for controlling these weeds.
Finally, fields with a history of soilborne mosaic virus or wheat spindle-streak mosaic virus should be treated with special consideration. These two viruses are vectored by a soil-dwelling micro-organism. Of the two viruses, soilborne mosaic virus is the more damaging to yield. Once the virus and microorganism are present in a field, there are no practical ways to eliminate them. There are excellent wheat varieties that have resistance to one or both of these diseases, and a field known to have one or both viruses should either be planted with an appropriately resistant variety or removed from small-grain production.
Seeding Rate, Drill Calibration, and Drill Operation
A good stand of wheat is the best defense against weeds and cereal leaf beetle and is the best indicator of a high yield potential. Although cereal leaf beetles are attracted to thicker stands of wheat, wheat can compensate better for cereal leaf beetle feeding in thicker stands than thinner stands. When planting on time with high quality seed into conventionally tilled seedbeds, the target seeding rate is 30 to 35 seeds per square foot. Increase this target rate if seed germination is below 90%, or if planting more than two weeks after the dates shown in Figure 4-1. A complete guide to seeding rate, drill calibration, planting depth, and other planting considerations can be found in “Small Grain Seeding Rates for North Carolina” in the Small Grain Production Guide (AG-850, revised).
Special Considerations for Broadcast Seeding
Broadcast seeding often results in uneven seed placement in the soil, which results in uneven emergence and stands. Seeds may be placed as deep as 3 to 4 inches, where many seeds will germinate but not emerge through the soil surface. Other seeds may be placed very shallow or on the soil surface. These seeds often do not survive due to dry soil or winter damage. The uneven stands from broadcasting often result in lower yields compared with drilling. Uneven seed depth and reduced yields are especially problematic when incorporated with a disk. Most growers who have been successful with broadcast seeding use a special implement (such as a Dyna-Drive) that allows tillage to a specified depth. Because plant establishment potential is reduced and seed placement is not uniform, seeding rates should be increased for broadcast seeding. Increase broadcast seeding rates by 30% to 35% percent over drilled seeding rates.
Soil Fertility
Soil pH is very important for a high-yielding wheat crop. Low soil pH can result in poor growth and development. High soil pH, especially on coarse-textured soils, can result in manganese deficiencies. Wheat that yields 65 bushels per acre takes up about 45 pounds of phosphate per acre (most of which is removed with the grain) and about 135 pounds of potash per acre (of which about 100 pounds is in the straw). Wheat is a moderately heavy feeder, but not as heavy as corn. For best yield results, an organically approved nitrogen source (such as manure, compost, or a tilled-in legume) should be added at or before planting and again in the spring. A wheat crop yielding 65 bushels per acre will take up about 70 pounds of nitrogen per acre. See Chapter 9 of this guide for more information on soil fertility in organic production. Some organic farms are demonstrating large nitrogen (N) carryover and may not need any spring nitrogen. Unfortunately, no soil test exists that will predict how much nitrogen carryover to expect. In early spring, it is possible to tissue test a wheat crop and determine how much additional nitrogen, if any, is needed to produce optimal yield. Information about how to use this tissue test can be found in “Nitrogen Management for Small Grains” in the Small Grain Production Guide (AG-850, revised).The application window for spring N is very narrow, and the source of fertility affects whether the nitrogen will be released in time to maximize yield. For manure sources, only the ammonium fraction should be considered available in time. This fraction is labeled “Ammonium NH4+-N” on the waste analysis reports from the North Carolina Department of Agriculture. Some of the slower releasing forms of nitrogen in the manure may be available during grain fill when protein content is set.
Weed Management
Essentially all weed control in organic wheat must be achieved in seedbed preparation before planting. Little to no cultivation is used in wheat after planting to kill emerging weeds, but a rotary hoe or tine weeder can be used before the crop emerges and again at the one-to-three leaf stage. However, weeds usually cause fewer problems in wheat than in corn or soybeans because wheat is a strong competitor against weeds and is drilled in narrow rows that quickly shade the soil. This is especially true of wheat that is planted near the dates shown in Figure 4-1. Most wheat drills are set to plant rows that are 6 to 8 inches apart. Organic producers may want to take advantage of row spacing as narrow as 4 inches to help the wheat outcompete winter annual weeds. Avoid planting organic wheat in fields with Italian ryegrass or wild garlic as these weeds can lead to quality problems in the harvested grain. Also, use caution with hairy vetch as a cover crop in fields where wheat will be planted because hairy vetch that reseeds can contaminate wheat grain with seeds that are similar in size and weight and thus are difficult to separate. See Chapter 7 for more information on weed management in organic production systems.
Insect Pest Management
Many kinds of insects can be found in wheat fields, but only a few are likely to threaten yield. A detailed description of small grain insect pests and their management can be found in the “Insect Pest Management for Small Grains” chapter of the Small Grain Production Guide (AG-850, revised).Organic producers should take note of the following potential insect pests:
Aphids
Aphids are small sucking insects that colonize small grains early in the season and may build up in the spring or fall. They injure the plants by sucking sap or by transmitting barley yellow dwarf virus (BYDV). Aphid populations are usually kept in check by weather conditions (such as freezing temperatures in late fall) and biological control agents, such as lady beetles, parasitic wasps, syrphid fly maggots, and fungal pathogens, which are often abundant in small grains. Consequently, direct yield reductions due only to aphid feeding are rare. On the other hand, BYDV can be a serious problem, especially when it is transmitted to wheat plants in the fall. Because cold temperatures kill aphids, planting near or after the first freeze (Figure 4-1) is a good way to avoid early aphid feeding and BYDV infections. If BYDV has been a problem in the past, selecting wheat varieties that are resistant to it may also be valuable.
Armyworm
Armyworm infests small grains, usually wheat, from late April to mid-May. They can cause serious defoliation, injury to the flag leaf, and head drop. No cultural management options are available for armyworm. The reason for this is that populations are sporadic in both space and time. When infestations do occur, populations tend to be extremely high, defoliating leaves and sometimes clipping heads. For this reason, curative measures are recommended. Organic growers have the choice of accepting the feeding of armyworms or using an insecticide approved for organic production (such as a spinosad or pyrethrin) in emergency situations.
Cereal Leaf Beetle
The cereal leaf beetle has one generation each year, and both the adult and larval stages eat leaf tissue on wheat and oats. They also feed on barley, triticale, and rye. Leaf-feeding by larvae during April and May can reduce yields. Cereal leaf beetle adults are attracted to thickly tillered wheat fields, but larval feeding is more apparent in thinner wheat because the larva-to-tiller ratio is higher in thinner wheat. Management practices that lead to densely tillered stands by mid-February can help to reduce the risk of too much tissue loss from cereal leaf beetle feeding as the additional tillers can compensate for the tissue lost from feeding. These practices include planting on time, using high quality seed planted at recommended seeding rates, making sure that preplant fertility is adequate for rapid fall growth, and applying a split nitrogen application in February and March if additional tillering is needed in the spring. Note that nitrogen does not influence the cereal leaf beetle population, but the increased tillers can increase the compensatory ability of the wheat stand. Insecticides approved for organic production (such as a spinosad or pyrethrin) and labeled for cereal leaf beetle may be applied in emergency situations. However, while spinosad will provide adequate control of light infestations, it will not provide adequate control when cereal leaf beetle populations are high.
Hessian Fly
In recent years, numerous North Carolina fields have suffered extensive losses because of Hessian fly infestations. Historically a wheat pest in the Midwest, changes in field-crop production including early-planted cover crop wheat, increased adoption of no-tillage double-cropped soybeans, and the use of wheat as a cover crop for strip-tillage cotton and peanut production have permitted the Hessian fly to reach major pest status in North Carolina. Organic farmers should use several methods to minimize Hessian fly problems.
- Because the Hessian fly life cycle depends largely upon the presence of wheat stubble, using rotations that do not plant new wheat into or near a previous wheat crop’s stubble will be the most effective way to prevent infestations. Additionally, since the Hessian fly is a weak flier, putting at least one field (or about ¼ mile) between new wheat plantings and the previous season’s wheat fields can be a successful method of preventing new infestations.
- Disking wheat stubble after harvest effectively kills Hessian fly. Burning is not as effective as disking. Although burning wheat straw will reduce populations, many pupae will survive below the soil surface.
- Serious Hessian fly infestations have occurred in areas where wheat for grain was planted near early-planted wheat for cover or early-planted wheat for dove hunting purposes. In organic systems using cover crops, selecting a small grain other than wheat will reduce Hessian fly populations. Oats, rye, and triticale are not favorable for Hessian fly reproduction and do not serve as a nursery.
- In many wheat-producing regions, a “fly-free date” has been established to guide growers in planting after the first freeze has killed the Hessian fly adults. This approach has not worked in North Carolina because our first freeze is highly unpredictable and may not occur until it is too late to plant. Instead, it is a good idea to plant wheat on or after the dates in Figure 4-1.
- If Hessian fly pressure is anticipated, selection of wheat varieties resistant to Hessian fly is a good idea.
Disease Management
The best disease management tactic for organic producers is to avoid diseases in the first place by selecting wheat varieties with good resistance packages. When planning for an organic small grain crop, variety selection and cultural practices should include consideration of the following diseases.
Barley Yellow Dwarf Virus (BYDV)
As cold temperatures kill the aphids that vector BYDV in the fall, planting near or after the first freeze (Figure 4-1) is a good way to avoid BYDV infections. Avoid planting into unincorporated light-colored residues of corn or other crops, as these attract aphids.
Powdery Mildew
One of the most yield-limiting factors in North Carolina wheat production is powdery mildew. This is especially true in the coastal plain, the southern piedmont, and some tidewater areas. Conventional producers often do not consider powdery mildew in their planning because they can rely on foliar fungicides to control the disease if it occurs, but organic producers do not have that luxury.
- The best protection against powdery mildew is to select wheat varieties that are resistant to it. Organic producers in the coastal plain who want high-yielding wheat must plant mildew-resistant varieties. Variety resistance to powdery mildew may break down over time, so organic producers should check the most recent variety ratings every year before ordering seed.
Leaf Rust
Leaf rust is a foliar disease that attacks wheat late in the growing season; some leaf rust can therefore be tolerated. While leaf rust can occur anywhere in North Carolina, it is most likely to be a problem in the coastal plain and tidewater regions. Conventional producers rely on foliar fungicides to protect the crop from this disease. Organic producers must select varieties with at least some resistance to leaf rust (a rating of moderately susceptible, moderately resistant, or resistant). Organic producers, especially those in the coastal plain, should try to select varieties that have a combination of powdery mildew and leaf rust resistance. As with powdery mildew, variety resistance to leaf rust also deteriorates from year to year, so organic producers should check the most recent variety ratings annually before ordering seed.
Loose Smut
Loose smut symptoms occur between heading and maturity. Infected seed appears normal. The fungus, which is found inside the embryo of the seed, will grow within the plant from germination until the seed heads emerge and smutted grains appear. Therefore, symptoms from an infection that occurs in one year will not be seen until plants from the infected seed mature in another year. Because loose smut is seedborne, control measures focus on the seed to be planted. Certified seed fields are inspected for loose smut, and strict standards are enforced. Seed from fields with loose smut are rejected. Therefore, using certified seed is a highly effective way to avoid loose smut. Organic producers who use farmer-saved seed should never plant seed from a crop infected with loose smut.
Septoria Nodorum Blotch (SNB)
Septoria nodorum blotch (SNB), sometimes called glume blotch, is caused by the fungus Parastagonospora nodorum, and can be a serious disease of wheat. Symptoms may occur at any time during the plant’s growth and on any portion of the plant.
- Because wheat residues harbor the fungus, unincorporated residues can produce a severe SNB epidemic if fungal spores are splashed up onto the new crop. This puts no-till planted wheat that follows directly behind double-cropped soybeans at higher risk of an SNB epidemic. Conversely, plowing under wheat stubble will eliminate residue as a source of infection.
- When SNB gets onto the developing grain head, the grain may be infected. If this grain is planted, the seedlings may be infected with SNB. Consequently, SNB can be seedborne. Using certified seed should help minimize SNB. Organic farmers should never save seed for planting if the wheat crop has had a serious SNB epidemic.
Stripe Rust
The Mid-Atlantic states experience a significant wheat stripe rust epidemic only once every four or five years. Stripe rust is caused by the fungus Puccinia striiformis, which does not appear to overwinter here, so spores generally must blow in from points south and west. Severe stripe rust epidemics usually start earlier than leaf rust, however, and the disease multiplies quickly, so it can be devastating to a susceptible variety.
Fusarium Head Blight (Scab)
In the U.S., Fusarium head blight (FHB, or head scab) of small grains is mainly caused by the fungus Fusarium graminearum, which also infects corn. Scab can occur in all small grains. Wheat and barley are the most susceptible to the disease, oats are a little less susceptible, and rye and triticale are the most resistant. Infection occurs at or soon after flowering, when fungal spores reach small-grain heads by wind or rain-splash. Once established in a spikelet, the fungus can spread through the rachis, or central stem of the head, to other spikelets, resulting in heads that are partly green and partly blighted or bleached. Superficial pink or orange spore masses can sometimes be seen on infected spikelets. Early infections can cause kernel abortions, and later infections can cause shriveled kernels (“tombstones”) that have low test weight. Scab produces toxins in the harvested grain, the most common being DON (deoxynivalenol, or vomitoxin). When DON reaches 2 parts per million (ppm), the grain is no longer fit for human consumption and cannot be sold to a flour mill. When DON reaches 5 ppm, the grain is no longer fit for swine feed; feed for poultry and cows has a higher DON tolerance. Wet weather before, during, and soon after small-grain flowering is the main factor determining whether there is a severe head scab epidemic. Warm temperatures (59°F to 86°F) before and during flowering also favor scab. Sadly, no single management practice will defeat scab. However, wheat producers who take the following measures will reduce the likelihood of a major scab problem.
- Many wheat varieties have moderate scab resistance (Table 4-1). There are also a few barley varieties with moderate scab and DON resistance (such as Endeavor, LCS Calypso, SU Mateo, and Violetta).
- Spring weather is often not warm and wet for more than a week or two. Scab risk can be reduced by planting at least two wheat varieties from different heading-date classes (for example, one medium variety and one late variety). This way, head emergence and flowering will be staggered through the spring, reducing the chance that environmental conditions will be conducive to scab in all wheat fields. A second way to force wheat to flower at different times in the spring is to stagger planting dates.
- Scout for scab before grain heads turn golden, when the contrast between the bleached and green parts of heads is still apparent. If scab is severe (more than 10% of heads), increase the combine fan speed so that the lightweight diseased grain is blown out the back along with the chaff. This will not remove all the infected grain but can help reduce mycotoxin levels in grain heading to market.
For an organic producer, variety resistance is the only line of defense. Variety ratings for Fusarium head blight are given in Table 4-1 and on the NC State Extension Organic Commodities website.
| Wheat Variety (from 2022 NC Official Variety Trial) | FHB (scab) rating |
|---|---|
| AgriMAXX 473 | MR |
| AgriMAXX 481 | MR |
| AgriMAXX 492 | MS |
| AgriMAXX 502 | MS |
| AgriMAXX 503 | MS |
| AgriMAXX 505 | MS |
| AgriMAXX 513 | MR |
| AgriMAXX 514 | MS |
| AgriMAXX 516 | MS |
| AgriPro 1947 | S |
| AgriPro 1950 | MS |
| AgriPro 1971 | MS |
| AgriPro 1987 | MR |
| AGS 2024 | S |
| AGS 3040 | MR |
| CG 509 | S |
| CG 55 | MS |
| CG Leslie | MS |
| CP8045 | MS |
| CP8118 | S |
| DG 9002 | MS |
| DG 9070 | MS |
| DG 9120 | MR |
| DG 9151 | MR |
| DG 9172 | MR |
| DG 9701 | MR |
| DG 9811 | MS |
| Featherstone 125 | MS |
| FS 601 | MS |
| FS 624 | S |
| FS 875 | MS |
| FS 878 | MR |
| FS 891 | MS |
| KWS 263 | S |
| KWS 291 | S |
| Laverne | MS |
| Liberty 5658 | MS |
| LW2026 | S |
| LW2068 | MS |
| LW2148 | MS |
| LW2169 | MR |
| LW2848 | MS |
| LW2958 | MS |
| NSS1450 | MR |
| NSS1472 | MS |
| SH 4400 | MS |
| SH 7200 | MS |
| SH 9310 | MS |
| SH 9520 | S |
| Shirley | S |
| SW55SR | MS |
| SW65SR | MS |
| Syngenta 547 | MS |
| Syngenta Richie | S |
| Syngenta Viper | MS |
| USG 3118 | S |
| USG 3230 | MS |
| USG 3329 | MR |
| USG 3352 | S |
| USG 3451 | MS |
| USG 3458 | S |
| USG 3536 | MS |
MR=Moderately resistant, MS=Moderately susceptible, S=Susceptible ↲
Diagnoses and Assistance from the Plant Disease and Insect Clinic
If you have a question about whether a small-grain problem is caused by a disease, an insect, or something else, send a sample to NC State’s Plant Disease and Insect Clinic for diagnosis. Send whole affected plants with intact roots surrounded by moist soil. Place a plastic bag around the roots to ensure they remain moist, but do not encase the tops in plastic. If the plants are tall, it’s fine to bend them double. For instructions and a submission form, contact the Plant Disease and Insect Clinic by phone or mail:
Plant Disease and Insect Clinic
NC State University
Campus Box 7211
1227 Gardner Hall, 100 Derieux Place
Raleigh, NC 27695-7211
For disease problems: 919.515.3619
For insect problems: 919.515.9530
Email: plantclinic@ces.ncsu.edu
Publication date: March 19, 2024
AG-660
Other Publications in North Carolina Organic Commodities Production Guide
- Chapter 1: Introduction
- Chapter 2: Organic Crop Production Systems
- Chapter 3: Crop Production Management - Corn
- Chapter 4: Crop Production Management - Wheat and Small Grains
- Chapter 5: Crop Production Management - Organic Soybeans
- Chapter 6: Crop Production Management - Flue-Cured Tobacco
- Chapter 7: Crop Production Management - Peanuts
- Chapter 8: Crop Production Management - Sweetpotatoes
- Chapter 9: Soil Management
- Chapter 10: Weed Management
- Chapter 11: Rolled Cover Crop Mulches for Organic Corn and Soybean Production
- Chapter 12: Organic Certification
- Chapter 13: Marketing Organic Grain Crops and Budgets
- Chapter 14: Organic Market Outlook and Budgets
- Chapter 15: Resources for More Information on Organic Commodity Production
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.