In a hungry and increasingly competitive world, reducing postharvest food losses is a major agricultural goal. For highly perishable commodities, such as tomatoes, squash, and peaches, as much as 30 percent of the harvested crop may be lost to postharvest diseases before it reaches the consumer. Investments made to save food after harvest are usually less costly for the grower and the consumer and less harmful to the environment than efforts to increase production. Even a partial reduction in postharvest losses can significantly reduce the overall cost of production and lessen our dependence on marginal land and other scarce resources.
Many factors contribute to postharvest losses in fresh fruits and vegetables. These include environmental conditions such as heat or drought, mechanical damage during harvesting and handling, improper postharvest sanitation, and poor cooling and environmental control. Efforts to control these factors are often very successful in reducing the incidence of disease. For example, reducing mechanical damage during grading and packing greatly decreases the likelihood of postharvest disease because many disease-causing organisms (pathogens) must enter through wounds.
Chemicals have been widely used to reduce the incidence of postharvest disease. Although effective, many of these materials have been removed from the market in recent years because of economic, environmental, or health concerns.
Increased interest in the proper postharvest handling of fresh fruits and vegetables in North Carolina has prompted the widespread use of flumes, water dump tanks, spray washers, and hydrocoolers. To conserve water and energy, most postharvest processes that wet the produce recirculate the water after it has passed over the produce. This recirculated water picks up dirt, trash, and disease-causing organisms. If steps are not taken to prevent their spread, these organisms can infect all the produce that is subsequently processed. In the past, various fungicides and bactericides have been used (alone or in combination with chlorination) to prevent the transmission of diseases. These materials have often been favored over chlorination because they provide some residual protection after treatment.
At present, chlorination is one of the few chemical options available to help manage postharvest diseases. When used in connection with other proper postharvest handling practices, chlorination is effective and relatively inexpensive. It poses little threat to health or the environment. This publication has been prepared to acquaint growers, packers, and shippers with the proper use of chlorination.
Postharvest Diseases
Many types of postharvest disorders and infectious diseases affect fresh fruits and vegetables (Table 1). Disorders are the results of stresses related to excessive heat, cold, or improper mixtures of environmental gases such as oxygen, carbon dioxide, and ethylene. Some disorders may be caused by mechanical damage, but all are abiotic in origin (not caused by disease organisms) and cannot be controlled by chlorination or most other postharvest chemicals. However, abiotic disorders often weaken the natural defenses of fresh produce, making it more susceptible to biotic diseases, those that are caused by disease organisms. Further, in many cases injuries caused by chilling, bruising, sunburn, senescence, poor nutrition, and other factors can mimic biotic diseases.
| Commodity and Disease | Pathogens* |
| Apples | |
| Blue mold | Penicillium expansum (f) |
| Gray mold | Botrytis cinerea (f) |
| Black rot | Physalospora obtusa (f) |
| Bitter rot | Glomerella cingulata (f) |
| Grapes and small fruit | |
| Blue mold | Penicillium sp. (f) |
| Gray mold | Botrytis cinerea (f) |
| Rhizopus rot | Rhizopus stolonifer (f) |
| Potatoes | |
| Fusarium tuber rot | Fusarium spp. (f) |
| Wet rot | Pythium sp. (f) |
| Bacterial soft rot | Erwinia spp. (b) |
| Slimy soft rot | Clostridium spp. (b) |
| Peaches and plums | |
| Brown rot | Monilinia fructicola (f) |
| Rhizopus rot | Rhizopus stolonifer (f) |
| Gray mold | Botrytis cinerea (f) |
| Blue mold | Penicillium sp. (f) |
| Alternaria rot | Alternaria sp. (f) |
| Gilbertella rot | Gilbertella persicaria (f) |
| Sweetpotatoes | |
| Bacterial soft rot | Erwinia chrysanthemi (b) |
| Black rot | Ceratocystis fimbriata (f) |
| Ring rot | Pythium spp. (f) |
| Java black rot | Diplodia gossypina (f) |
| Fusarium surface rot | Fusarium oxysporum (f) |
| Fusarium root and stem rot | Fusarium solani (f) |
| Rhizopus soft rot | Rhizopus nigricans (f) |
| Charcoal rot | Marcrophomina sp. (f) |
| Tomatoes and peppers | |
| Alternaria rot | Alternaria alternata (f) |
| Buckeye rot | Phytophthora sp. (f) |
| Gray mold | Botrytis cinerea (f) |
| Soft rot | Rhizopus stolonifer (f) |
| Sour rot | Geotrichum candidum (f) |
| Bacterial soft rot | Erwinia spp. (b) or Pseudomonas spp. (b) |
| Ripe rot | Colletotrichum sp. (b) |
| Vegetables (general) | |
| Watery soft rot | Sclerotinia sp. (f) |
| Cottony leak | Pythium butleri (f) |
| Fusarium rot | Fusarium sp. (f) |
| Bacterial soft rot | Erwinia sp. (b) or Pseudomonas spp. (b) |
| * (f) = fungus, (b) = bacterium | |
The control of biotic postharvest diseases depends on understanding the nature of disease organisms, the conditions that promote their occurrence, and the factors that affect their capacity to cause losses. Postharvest diseases may be caused by either fungi or bacteria, although fungi are more common than bacteria in both fruits and vegetables. Postharvest diseases caused by bacteria are rare in fruits and berries but somewhat more common in vegetables. Viruses seldom cause postharvest diseases, although they, like postharvest disorders, may weaken the produce.
Most postharvest fungal diseases (rots) are caused by the dispersion of tiny dustlike spores formed by the actively growing pathogen. Spores have adaptations that allow them to survive in hot, cold, or very dry conditions. They may be carried great distances by wind or water and can cover most exposed surfaces in great numbers.
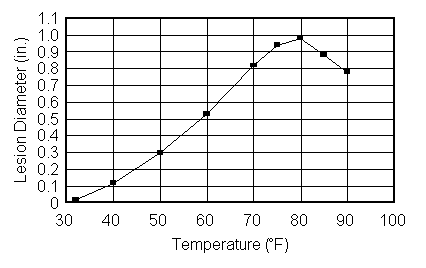
Spores may remain dormant for long periods until the correct conditions for their germination and growth occur. These conditions include the presence of water (in liquid form or as high relative humidity), warm temperatures, low light levels, adequate levels of oxygen and carbon dioxide, and the presence of nutrients in the form of sugars, starches, or other organic compounds. Many immature fruits and vegetables contain compounds that inhibit the growth of some disease organisms. These compounds and the resistance they provide are often lost during ripening. Therefore, a fresh wound on the surface of a warm, wet, ripened fruit or vegetable enclosed within a shipping container provides an ideal site for postharvest pathogens to colonize and develop. Gentle handling to prevent wounding and thorough cooling immediately after harvest can significantly reduce the incidence of postharvest disease. Figure 1 illustrates the effects of temperature on the development of brown rot (Monilinia fructicola) in peaches.
Spores of postharvest fungal pathogens are most susceptible to chemical control while they are germinating to produce actively growing mycelium. Under the right circumstances, germination can be rapid, often taking only a few hours. Once active growth is under way and the organism moves below the surface of the fruit or vegetable, chemical control becomes very difficult.
The potential for inoculation and infection by postharvest pathogens is always present when handling fresh produce. Understanding the several different ways by which these organisms can come into contact with the produce can be helpful in formulating control measures.
Soil and Field Conditions
Soil and decaying plant material in the field can contain postharvest pathogens in great abundance. Hard rains and wind can splash and distribute these materials onto unharvested produce. In addition, warm rainy conditions greatly favor the development of diseases in the field.
Contaminated Water
Water from ponds and streams should not be used for postharvest cooling, fluming, and washing. Water from these sources is often contaminated by runoff from fields and packing houses and may therefore contain large concentrations of postharvest pathogens. Using pond or stream water for irrigation can also contaminate produce in the field. Muddy water or water taken from the bottom of the pond is especially likely to be contaminated. Always use potable water from a well or other reliable supply.
Poor Packing House Sanitation
Pathogens brought into the packing house along with the produce will quickly contaminate all working surfaces. Disease-causing organisms will remain viable for months on surfaces such as tank walls, grading belts, and brushes. Wash all produce-handling equipment daily to remove dirt and decayed produce, and disinfect it with a strong chlorine solution on a regular schedule. Keep the packing house and the immediate vicinity clear of any overripe or rotting produce. Remove culls from the packing house and its vicinity immediately.
Air
Even the most meticulous attention to sanitation may not completely prevent contamination of fresh produce by disease organisms. Pathogens are present in the air and will infect produce under suitable circumstances. The best defense against airborne pathogens is sanitation, consistent chlorination, proper handling of the commodity, and quick and thorough cooling.
Although the skin of fruits and vegetables offers considerable protection against infection, pathogens can enter produce through a variety of openings when the produce is wetted. Various wounds, such as punctures, cuts, and abrasions, as well as stems and stem scars provide potential points of entry. The probability of a pathogen entering the produce increases with the size of the opening, the depth of submergence, the length of time the produce is in the water, and the water temperature. Even tiny natural openings (such as stomata and lenticels) can serve as pathways for disease organisms. A small amount of detergent added to the solution lowers the surface tension, increasing the ability of the chlorine to move into the small openings and destroy the pathogens.
A chlorine concentration of about 55 to 70 ppm at a pH of 7.0 is recommended for sanitizing most fruits and vegetables. A higher concentration may be needed if the pH is higher or if the temperature of the solution is more than 80°F. In actual practice, concentrations of up to 150 ppm of free chlorine have been recommended.
The Chemistry of Clorination
Chlorine is a very irritating, heavy, greenish yellow gas with a strong, pungent odor. Free chlorine is very reactive, combining with any chemical that will react with oxygen, and is never found uncombined in nature. Chlorine in the gaseous form is a very potent disinfectant, although it is seldom used in that form. It is much safer and easier to use when dissolved in water. Disinfection of produce using chlorine or some other chemical is nearly always done during hydrocooling or during the process of washing the produce to remove soil. Chlorine for disinfection may be obtained from one of three sources: pressurized chlorine gas, calcium hypochlorite (a soluble solid), or a solution of sodium hypochlorite.
Chlorine Gas
Chlorine gas is produced by the electrolysis of salt solutions (principally NaCl) and is furnished commercially in pressurized metal cylinders. Chlorination is accomplished by bubbling a metered amount of the gas into the supply water. Because of dangers involved with the use of chlorine gas and the expense of the metering equipment, the use of the gaseous form for chlorination is usually limited to large applications. Most municipal water supplies are disinfected with chlorine gas. The limited amount of chlorination required by most postharvest fruit and vegetable operations makes the use of chlorine gas impractical.
Calcium Hypochlorite
The most common source of chlorine used in postharvest chlorination is calcium hypochlorite. It is available commercially in the form of either a granulated powder or large tablets. Most commercial formulations are 65 percent calcium hypochlorite, with the balance consisting of stabilizers and inert materials. Calcium hypochlorite is relatively stable as long as it is kept dry, and it may be stored for extended periods. The property that makes it stable also makes it difficult to dissolve completely in water. Adding granulated calcium hypochlorite directly to the water often results in undissolved particles that adhere to the produce, causing undesirable bleaching and chlorine burns. This problem is particularly common in hydrocoolers because calcium hypochlorite is very slow to dissolve in cold water. Therefore, always dissolve granulated calcium hypochlorite in a small quantity of tepid water before adding it to the wash tank or hydrocooler. Calcium hypochlorite may be obtained in tablets that are added directly to the hydrocooler or wash tank to eliminate the problem of chlorine burns. Properly used, the tablets will dissolve slowly to yield a continuous supply of chlorine to the water. However, the tablets must be positioned carefully to ensure proper mixing of the chemical with the water.
Sodium Hypochlorite
The active ingredient of most liquid household bleaches, sodium hypochlorite is commonly used when the scale of postharvest chlorination is limited. Sodium hypochlorite is not generally available in solid form because it is difficult to store. It absorbs moisture readily from the atmosphere, causing it to release chlorine gas.
Household bleach is usually marketed as a solution of water and 5.25 percent sodium hypochlorite. Larger containers of 12.75 percent or 15 percent sodium hypochlorite solutions are also available through some laundry and swimming pool chemical suppliers. For the same amount of chlorination, a sodium hypochlorite solution is generally more expensive than granular calcium hypochlorite because of the additional shipping and handling costs associated with the water it contains.
Chlorination chemicals can be added to the water manually, or concentrated solutions of sodium or calcium hypochlorite can be injected into the wash tank or hydrocooler at a continuous and measured rate. Commercial chlorine injector systems are particularly useful in operations where a continuous supply of clean, chlorinated water is required. Injector systems consist of a feed tank and an electrically operated pump with a variable output. Chlorine injectors should always be isolated from water supply lines with an approved check valve to prevent backflow into the fresh water system.
Chlorination Effectiveness
Chlorination is a dynamic chemical process. Its effectiveness is influenced by a number of factors. Proper chlorination requires frequent monitoring of the solution and a thorough understanding of the factors involved. These factors include the pH of the solution, chlorine concentration, water temperature, amount of organic matter present, exposure time, and the growth stage of the pathogens present.
Solution pH
The pH of a solution is a measure of its acidity or alkalinity. A solution that is neutral (neither acid nor alkaline) has a pH of 7.0. Solutions with pH numbers less than 7.0 are acid; the lower the number, the greater the acidity. On the other hand, the greater the number above 7.0, the more alkaline the solution. A change of one pH unit indicates a ten-fold change in acidity or alkalinity.
The pH of the solution has a significant effect on the level of chlorination activity. When chlorine gas or one of the hypochlorite salts is added to water, each will generate chlorine gas (Cl2), hypochlorous acid (HOCl), or hypochlorite ions (OCl) in various proportions, depending on the pH of the solution. The form desired for chlorination is hypochlorous acid (HOCl). Hypochlorite ions are relatively inactive, and chlorine gas quickly bubbles out of the solution, causing worker discomfort and serving no useful purpose.
At a pH slightly above neutral, half of the chlorine will be in the form of hypochlorous acid and the other half in the form of hypochlorite ions. Very little will be in the gaseous form. Solutions that are more acid have a higher percentage of hypochlorous acid but are very unstable, allowing more of the chlorine to escape from the solution as a gas. To maximize the proportion of hypochlorous acid and hence the effectiveness of the solution, the pH should be kept in the practical range between 6.5 and 7.5.
Because well water in North Carolina varies from moderately acid to moderately alkaline, the pH should be checked with a pH meter or test papers before and after the chemicals are added and frequently during operation. Furthermore, even if the water initially has a near-neutral pH, the addition of hypochlorites will change the pH.
Different sources of chlorine have different effects on pH:
- Chlorine gas decreases pH
- Sodium hypochlorite increases pH
- Calcium hypochlorite increases pH slightly.
It may be necessary to add a common acid (like vinegar) to lower the pH. Small amounts of sodium hydroxide (lye) may be used to raise the pH. Inexpensive test papers for checking both the chlorine level and pH may be obtained from most swimming pool and chemical supply houses.
Chlorine Concentration
The concentration of a small amount of chemical in a solution is measured in units of parts per million (ppm). In the case of chlorination, this unit of measure indicates the number of parts of available chlorine, by weight, that there are in a million parts of solution. The quantity of calcium or sodium hypochlorite that must be added to a certain quantity of water to obtain a given concentration depends on
- the available chlorine content of the compound
- the concentration of the compound
- the volume of water to be treated.
Table 2 shows the minimum chlorine concentrations needed to kill all pathogens within one minute at two different temperatures, assuming a neutral pH. Table 3 gives the amount of 5.25 percent solution hypochlorite solution that must be added to 100 gallons of water to obtain various chlorine concentrations from 25 to 150 ppm. Table 4 gives the same information fo 65 percent calcium hypochlorite granules.
| Chlorine Concentration (ppm) | ||
| 77°F | 104°F | |
| Fungi | 30-40 | 10 |
| Bacteria | 20 | 10 |
| Pints of solution per 100 gallons of water | Approximate chlorine concentration (ppm) |
| 0.4 | 25 |
| 0.8 | 50 |
| 1.2 | 75 |
| 1.6 | 100 |
| 2.0 | 125 |
| 2.4 | 150 |
| Ounces of granules per 100 gallons of water | Approximate chlorine concentration (ppm) |
| 0.5 | 25 |
| 1.0 | 50 |
| 1.5 | 75 |
| 2.0 | 100 |
| 2.5 | 125 |
| 3.0 | 150 |
Temperature
The activity of chlorine increases with the temperature of the solution. Unnecessarily warm solutions should be avoided, however, because the chlorine escapes into the air more rapidly as the temperature increases. On the other hand, in hydrocooling the combined effects of low temperature and high pH values reduce chlorination efficiency.
Organic Matter
Chlorine has a particular affinity for soil particles and organic matter. Chlorinating dirty produce therefore depletes the chlorine supply much faster than relatively clean produce. The amount of chlorine constantly decreases with chlorination reactions. The more organic matter (such as fruit, leaves, or soil) in the tank, the more chlorine will be lost. As a result, the chlorine level should be checked and adjusted hourly, especially when large loads of produce are being processed. Extremely dirty produce (such as sweetpotatoes) is commonly washed with clean water before it is placed into the chlorination tank.
Exposure Time
The effectiveness of chlorination depends greatly on the length of time the produce is exposed to the chlorine solution. Quick dips are much less effective than longer exposures. However, most of the sanitizing action of the chlorine is accomplished within the first several minutes of exposure. Prolonged exposure to strong chlorine solutions has been known to cause surface bleaching. Experience is the best guide to the correct combination of treatment time and chlorine concentration for the crop being processed.
Growth Stage of the Pathogen
Disease organisms may be either in the active vegetative form or in the form of spores. Chlorine will readily kill the vegetative form, but fungal spores are 10 to 1,000 times more difficult to kill. Therefore, chlorine treatment rarely eliminates all pathogens and sterilizes the surface of the produce. Many spores may remain on the surface to develop later should the opportunity arise. Further, chlorine kills only on contact, not systemically, and is effective only on exposed pathogens such as those suspended in water or those on the surface of produce; chlorine does not kill pathogens below the skin because it cannot contact them. Chlorination leaves no residual effect. Therefore, produce exposed to pathogens after treatment is susceptible to reinfection.
Wastewater Disposal
Chlorinated wastewater is customarily drained at the end of each work day or more often if circumstances dictate. This wastewater often contains sediment, pesticides, and other suspended matter. If it is discharged to a municipal wastewater treatment plant or to surface waters (canals, creeks, or ponds), regulatory agencies may consider it to be industrial wastewater. Land application of this material is normally permitted, but a nondischarge permit may be required. Operators may be required to obtain wastewater discharge permits.
If you plan to use chlorination, check with the local office of the North Carolina Department of Environment, Health, and Natural Resources to determine whether a permit is required. Illegal disposal of hydrocooler wastewater may result in a substantial penalty.
The Environmental Protection Agency (EPA) regulation of May 8, 1991, (40 CFR part 180) exempts calcium hypochlorite and chlorine gas from residue tolerance requirements when it is used before or after harvest on any raw agricultural commodity. Any amount can legally be used; however, excessive chlorination can damage equipment, injure the surface of fruits and vegetables, and waste money. In addition, the use of excessive amounts of chlorine may pose a worker health and safety hazard that is regulated by the North Carolina Department of Labor.
Practical Rules for Successful Chlorination
- If water is not necessary in the packing process, do not use it. Wetting the produce greatly increases the likelihood of damage by postharvest diseases. If the produce must be washed to remove soil, there is no alternative to wetting. Hydrocooling also necessitates wetting the produce, although other methods, such as forced-air cooling, may be a viable option in some cases. When water is necessary in packing lines (for example, in dumping tanks, flumes, or a hydrocooler), always treat it to reduce the risk of disease.
- Monitor the chlorine concentration and the condition of the water. Check the chlorine concentration and pH frequently using test papers or electronic equipment. Automatic chlorination equipment is available that will continually monitor the condition of the solution, add chlorine, and correct the pH. Also, monitor the water temperature.
- Avoid overexposure. Do not allow the produce to remain in contact with the solution longer than necessary. Check circulation patterns in chlorination tanks to eliminate dead spots.
- Change the water frequently. Chlorination efficiency is poor in dirty water. If necessary, wash very dirty produce with clean water before it comes into contact with the chlorinated water.
- Dispose of wastewater properly. Before installing chlorination equipment, plan how you will dispose of the wastewater. Land application of wastewater is normally allowed, but check to see if a permit is needed. Illegal disposal of chlorinated water could result in a substantial fine.
- Practice good sanitation. Hose off the packing equipment and floors daily; remove any dirt and trash that has settled in the chlorination tank. Sanitize the equipment with a spray solution composed of four pints of 5.25 percent sodium hypochlorite solution in 10 gallons of water. As an alternative, steam clean the equipment with an approved detergent. Do not allow culls or decayed produce to remain in or around the packing house.
- Protect workers. For their safety and comfort, workers must be protected from the chlorine fumes associated with excessively high levels of chlorine. If the amount of chlorine gas in the work area is great enough to cause worker discomfort, the amount of chlorine being used is well above that required for proper postharvest sanitation. If air monitoring equipment is not available, chlorine concentrations can be checked by asking a person who has not been desensitized by the odor to enter the work area. If he or she can smell the chlorine, the level is probably adequate. The concentration is too high if workers are continually irritated by the odor.
- Remember that chlorination will not solve all your problems. Even the best chlorination program may not be sufficient to prevent all postharvest decay. Prompt handling, proper sanitation, and rapid cooling should all be part of your postharvest disease management program. Produce infected in the field or otherwise damaged cannot be saved by chlorination.
For More Information
The following publications in this series on the postharvest cooling and handling of fresh produce are available:
AG 414-1, Proper Postharvest Cooling and Handling Methods
AG 414-2, Design of Room Cooling Facilities: Structural & Energy Requirements
AG 414-3, Forced-Air Cooling
AG 414-4, Hydrocooling
AG 414-5, Top and Liquid Ice Cooling
Sponsored by the Energy Division, North Carolina Department of Commerce, with State Energy Conservation Program funds, in cooperation with North Carolina State University. However, any opinions, findings conclusions or recommendations expressed herein are those of the authors and do not necessarily reflect the views of the Energy Division, North Carolina Department of Commerce.
Publication date: Aug. 1, 1993
AG-414-06
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.