Introduction
Soybean seedling diseases are caused by various soilborne fungi and fungal-like organisms. These diseases primarily result in stand loss, and in severe cases, can lead to significant yield reductions. Wet conditions shortly after planting favor the development of seedling diseases, making them more prevalent in poorly drained or compacted soils. Additionally, the fungal pathogens responsible for these diseases are opportunistic, often infecting stressed seedlings that are weakened by factors such as waterlogged soils, insect or nematode damage, and herbicide injury.
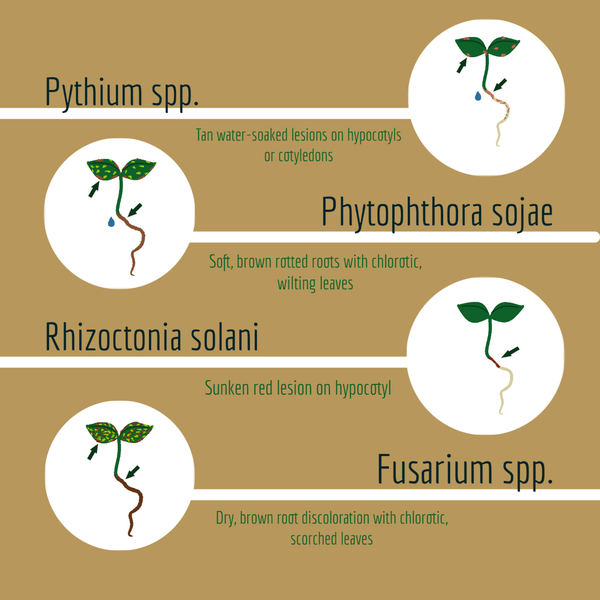
Commonly observed soybean seedling diseases include Fusarium root rot (Fusarium spp.), Rhizoctonia seedling blight (Rhizoctonia solani), Phytophthora root rot (Phytophthora spp.), and Pythium seedling blight (Pythium spp.). However, diagnosing the specific disease in the field can be challenging, as the symptoms associated with these seedling blights often resemble one another and the diseases can often occur together as a complex.
Symptoms, Favorable Conditions, and Infection
Fusarium Root Rot (Fusarium spp.)
Fusarium root rot is a common seedling disease caused by over 10 species of Fusarium. In North Carolina, the primary species responsible for this disease are Fusarium oxysporum and Fusarium acuminatum. Fusarium root rot can result in both pre- and post-emergence damping-off, leading to delayed emergence and stunting of infected seedlings. Root rot symptoms typically manifest as dark brown discoloration, with lesions extending through the vascular tissue to the hypocotyls and are often accompanied by the absence of secondary roots. Above-ground symptoms include chlorosis (yellowing) of the cotyledons, wilting, and the appearance of patchy areas in the field. Fusarium root rot can infect seedlings under a wide variety of environmental conditions and is often associated with stressful growing conditions.
Fusarium species have a broad host range, surviving in plant residue as mycelium and as chlamydospores (resilient resting spores) in the soil. Infections can occur when seeds or roots come into contact with these spores or mycelium. While Fusarium infections can take place at any point during the growing season, they are more likely to occur when plants are stressed or injured, as Fusarium species are opportunistic pathogens.
Rhizoctonia Seedling Blight (Rhizoctonia solani)
Rhizoctonia solani, the pathogen responsible for Rhizoctonia seedling blight, causes both pre- and post-emergence damping-off. Infected seedlings typically exhibit reddish-brown, sunken lesions that girdle the hypocotyl and lower stem at the soil line. This disease often appears in patches or affects entire sections of rows within the field. Like Fusarium root rot, this disease can also occur over a wide range of environmental conditions, preferring light and sandy soils and is also associated with stressed plants. However, prevalence tends to increase in warm and moist, but not saturated soils.
Rhizoctonia solani also has a broad host range that survives in plant residue as mycelium and in soil as sclerotia (overwintering fungal structure). Like Fusarium, infection can occur at any growth stage and also acts as an opportunistic pathogen when stress or injury to the plant occurs.
Phytophthora Root Rot (Phytophthora sojae)
Phytophthora root rot is primarily caused by Phytophthora sojae, although recent reports have identified another species, P. sansomena, as also contributing to the seedling disease. These pathogens belong to the oomycete group, a class of fungal-like, water-loving organisms. Infected soybeans often exhibit symptoms such as mushy, water-soaked stems at the seedling stage. Above-ground symptoms typically include wilting and stunting of plants, which are most commonly observed in patches, particularly in low-lying areas of the field. Phytophthora root rot can also develop under a wide variety of conditions, though it most commonly occurs in warm and saturated conditions, typically after heavy rainfall events where there is standing water.
Phytophthora sojae survives as oospores (resilient fungal-like spore) in plant residue or soil. The oospores germinate when soil is saturated and soil temps exceed 60°F. Mycelia from oospores can directly infect plants. The spread occurs when mycelia develop fungal structures (sporangia) that produce smaller, motile spores known as zoospores. Zoospores can move in water through soil and are attracted to seed and root exudates, causing the spores to germinate and penetrate plant roots.
Pythium spp.
Pythium species, causing Pythium seedling blight, are another group of oomycetes, therefore the disease symptoms are similar to Phytophthora root rot. Many species of Pythium have been identified to cause this seedling disease. Symptoms often include rotten, mushy seeds with poorly developed roots. Above ground symptoms may appear as water-soaked lesions on the hypocotyl and/or on the cotyledons and are most commonly observed in patches in low-lying areas of the field. Like Phytophthora root rot, Pythium seedling blight is most severe in poorly drained soils. Pythium can also infect across a wide range of soil temperatures typically preferring cool and wet conditions; however, some species are more prevalent in warm and wet conditions.
Like Phytophthora, Pythium species have a very similar survival mechanism and infection process. The overwintering oospores will germinate in saturated soils infecting the plant or producing more infectious spores that move through water to infect healthy plants.
Management
Cultural Practices
Planting soybeans in optimal environmental conditions that favor quick stand establishment of the seedling will reduce plant stress and mitigate disease risk. Improving soil drainage may also reduce stress and risk of infection. Optimal seeding rates and planting depths may also increase the rate of stand establishment to reduce the susceptibility of seedlings to various diseases. Crop rotation can help in some situations; however, this may not always be effective due to the wide host range of Fusarium and Rhizoctonia species and the longevity of Phytophthora and Pythium species.
Chemical Management
Scouting fields early is important to accurately diagnose and document disease history when determining which fungicide seed treatments are most effective on a specific disease. Not all seed treatments are effective for every seedling disease. For example, Pythium spp. and Fusarium spp. require different modes of action for disease prevention. Tools to help with scouting and seed treatment options can be found in the following links to publications found on the Crop Protection Network website:
Useful Resources
- The NC State University Plant Disease and Insect Clinic provides diagnostics and control recommendations
- The North Carolina Agricultural Chemicals Manual provides pesticide information for common diseases of North Carolina. The manual recommendations do not replace those described on the pesticide label, and the label must be followed.
Publication date: April 8, 2025
Reviewed/Revised: April 8, 2025
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.
NC Cooperative Extension prohíbe la discriminación por raza, color, nacionalidad, edad, sexo (incluyendo el embarazo), discapacidad, religión, orientación sexual, identidad de género, información genética, afiliación política, y estatus de veteran.
Recommendations for the use of agricultural chemicals are included in this publication as a convenience to the reader. The use of brand names and any mention or listing of commercial products or services in this publication does not imply endorsement by NC State University or N.C. A&T State University nor discrimination against similar products or services not mentioned. Individuals who use agricultural chemicals are responsible for ensuring that the intended use complies with current regulations and conforms to the product label. Be sure to obtain current information about usage regulations and examine a current product label before applying any chemical. For assistance, contact your local N.C. Cooperative Extension county center.
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.