An overview of processing mature sweet sorghum into ethanol and how to implement the process for a farm or residence
As gasoline prices continue a volatile and upward trend, many motorists are looking for alternatives to the increasing cost of fuel. Sweet sorghum is an attractive ethanol feedstock because it provides a directly fermentable source of aqueous sugar that can be relatively easy to process into fuel alcohol. Additionally, sweet sorghum is well-suited for the southeastern U.S. climate as it requires relatively low nutrient inputs, is drought-tolerant, and has a more or less 100-day production cycle that allows it to fit into many double-crop management rotations. This Cooperative Extension factsheet describes how to process mature sweet sorghum into ethanol and how this process could be implemented on a farm or private residence in the southeastern United States.
Sweet Sorghum Characteristics
Sweet sorghum is a sugar crop, similar to sugar cane and sugar beets, that may show promise as a source of sugar for ethanol fermentation (Nathan, 1978; Smith et al., 1987). It is an annual crop in the grass family. It is noted for its high photosynthetic efficiency, adaptability to temperate regions, and drought resistance (Nan and Ma, 1989; Worley et al., 1992; Hunter and Anderson, 1997; Gnansounou et al., 2005; Martini et al., 2006). Individual stalks can be over 10 ft tall with dry ton yields surpassing 10 tons/acre annually. The pith or stalks can be mechanically pressed to release a sugar juice (12% – 22% sugar) that can be filtered and directly fermented by yeast. The resulting ethanol can be separated through subsequent solids separation and distillation processes. The primary advantages of sweet sorghum over many other bioenergy feedstocks are the reduced processing steps and inputs required for complete conversion, which may reveal improved economic benefits (Worley et al., 1992). Challenges in using sweet sorghum juice include the harvest time, which is limited to three to four months per year, and maintenance of juice stability. A number of reports suggest that juice extraction should occur soon after harvest and processing needs to take place immediately (Gnansounou et al., 2005; Kundiyana et al., 2006). Because there are issues with juice transportation, storage, and stability, an opportunity exists for individual farms to generate their own fuel ethanol in small, decentralized systems. The information in this factsheet is based on research efforts that have focused on farm-based sorghum ethanol production (Bridgers et al., 2011; Bennett and Anex, 2008; Kundiyana et al., 2006).
Sweet Sorghum to Ethanol Overview
Sweet sorghum contains relatively high levels of sugar (12% – 20%) in the juice contained in the central portion of the stalk, known as pith. Squeezing the stalk will release these pith juices and provide a solution that is primarily made up of sugar and water. The extracted broth contains the sugar concentration and nutrients necessary to support ethanol fermentation by yeast without other additives (Bridgers et al., 2011; Rains et al., 1990). Simply squeeze out the juice, provide the correct environmental conditions, and inoculate the broth, and the ethanol production process will commence. Following the fermentation, an ethanol and water solution will be present. It is necessary to distill the solution and remove the water, thereby producing a more pure form of ethanol, suitable for fueling an engine. In the following sections, we describe the conversion of sweet sorghum juice to ethanol in greater detail.
Harvest and Juice Collection
Harvest Methods
Sweet sorghum has traditionally been manually harvested using machetes or axes to cut individual stalks. Harvesting sweet sorghum can be a difficult process as there are few mechanized systems to support this aspect of the production cycle. The stalks are then collected and moved to a central location for further processing. The University of Georgia has estimated that over $540/acre (~$926/acre in 2013 adjusting for inflation) is required to cover the manual labor costs (University of Georgia, 1991). A sickle bar mower could also be used to support cutting operations, and collection of stalks from the field could be aided with the use of a cart or wagon. The use of these mechanical tools would increase harvest efficiency and decrease costs.
An additional cost-effective method available for harvest is the use of fully-mechanized, traditional agricultural equipment, which has been demonstrated by our research group. Forage choppers can be used to harvest the standing sweet sorghum and chop it into small pieces. These stalk pieces can then be blown into and collected in a forage box mounted on a truck or towed by a tractor. The small stalk pieces can then be squeezed to extract juice.
Juice Expression and Collection
The oldest and most common means of juice extraction is passing whole stalks through a set of rollers (Figure 2). Studies using this type of press have shown that typically less than 50% of the juice is collected and a roller press relies heavily on manual labor. Additionally, the leaves should be removed from the stalks prior to crushing. The leaves do not contain sugar and simply add more water, thereby diluting the sugar juice. While this approach is labor intensive and has reduced efficiency issues, it is simple to implement and requires a low capital investment. Leaves can be stripped using a pitchfork to rake down standing stalks or removed manually from cut stalks, or a device for leaf stripping could be developed.
Another method for juice extraction that we have demonstrated is passing material harvested with a forage chopper through a screw press (Figure 3). Although a screw press is an expensive investment, it provides a significant improvement in terms of juice output and labor cost reductions. A screw press consists of a spinning auger that decreases the clearance between the auger flighting and
press structure as the material travels down the press. As this clearance decreases, more pressure is exerted on the sorghum material and juice is forced out. These machines are common in the citrus and fruit juice processing industries.
Juice Collection Considerations
As the juice (Figure 4) is extracted from the plant and exits the press, it is important to initiate some crude juice purification activities. Typically, this will involve removing as many nonsugar solids as possible, such as plant material, soil, and additional trash. A simple low-cost way to filter the juice is the use of cheesecloth. A set of metal screens is another viable filter mechanism. As the juice exits the press, it should be passed through the filter material to eliminate potential large solid contaminants.
Sorghum material can spoil very quickly. A very important factor to remember when handling whole stalks, chopped material, and raw sorghum juice is all three contain a sugary solution that is a preferred growing medium for many microorganisms. Use freshly squeezed juice as soon as possible to prevent other microorganisms from either consuming the sugars or creating an environment that will prevent the yeast from flourishing. Although stalk material is difficult to preserve, once the juice is extracted, short-term preservation methods such as refrigeration can be used to store the juice.
Fermentation
Creating the Proper Fermentation Environment
Fermentation involves the use of yeast (Saccharomyces cerevisiae) to consume and metabolize sugars from the sweet sorghum’s pith juice. As sugars are consumed by yeast, they are converted to ethanol and carbon dioxide. Yeasts are living organisms, so it is important to provide an environment that will support their growth and allow them to complete the conversion process. Simply adding yeast to a sugar juice and hoping for the best will not lead to the anticipated results. The following five criteria are critical for creating a suitable environment for ethanol fermentation:
- Sterility. The type of container is not that important. The important characteristic is that the container can be cleaned and left sterile after a fermentation run is complete. If the container is not sterile, there could be lingering microorganisms (such as bacteria and fungi) that will compete with the ethanol-producing yeast to generate ethanol. Dilute bleach solutions, soap solutions, and steam are common methods used to sterilize a fermentation container. If soap or another chemical is used, make sure residues are not left behind. These antimicrobial chemicals will kill both the unwanted germs as well as the yeast, so all chemical residues must be rinsed away. Additionally, make sure the material used in the construction of the fermentation vessel is capable of being thoroughly cleaned.
- Anaerobic. Once yeasts are added to the sugary pith juice from sweet sorghum, it is important to seal off all new sources of oxygen to the fermentation vessel. In initial growth stages, yeasts will consume the oxygen present in the juice and generate carbon dioxide that will displace oxygen in the vessel, maintaining a “no oxygen” environment and favoring the production of ethanol. It is important to remember that the carbon dioxide produced will fill the vessel with gas and an air lock will be needed. The air lock allows the carbon dioxide to escape, preventing a pressure buildup in the fermentation vessel. As the carbon dioxide escapes, oxygen is not allowed to re-enter the fermentation vessel. Air locks can be found on many recreational home brewing and wine making consumer websites.
- Temperature. Most yeasts perform best in an environment that has a temperature in the range of 90° to 105°F. On a summer day this temperature can be achieved with outdoor ambient temperatures. In the fall and winter months, however, it will be necessary to supply supplemental heat to the fermentation vessel. Circulating hot water around the fermentation vessel (i.e., a jacketed vessel) may be the easiest, most cost-effective approach. Another heating option would be direct heating of the fermentation vessel with a heating element. This, however, might lead to overheating that can inactivate the yeasts. If temperature is not controlled, production rates can fluctuate, the length of fermentation time increases, and incomplete use of sugars can occur (resulting in lower ethanol concentrations).
- pH. Yeast organisms perform best in an environment that has a near neutral pH, so pH values between 5.5 to 7.5 are best for the fermentation of sweet sorghum juice. Generally, the naturally expressed juice will be in this range. The pH value should be monitored during the fermentation process as dramatic changes in the value indicate the process has failed. For instance, a quick drop in pH to the 4 to 4.5 range is usually an indication that sterility measures failed and acid producing bacteria are present. However, small drops in pH over the course of a well-performed fermentation without control are common.
- Nutrients. As of March 2014, no evidence has been gathered to support the need for addition of nutrients to sweet sorghum juice for ethanol fermentation activities. Yeast nutrients are minerals, vitamins, and proteins that support growth and development if the fermentation medium is insufficient. Experience from our research as well as other efforts indicate that the addition of nutrients will not improve fermentation efficiency, mostly because the sorghum juice itself seems to contain enough of the needed nitrogen, potassium, phosphorous, and other macro- and micro-nutrients (Bridgers et al., 2011).
Fermentation Platform
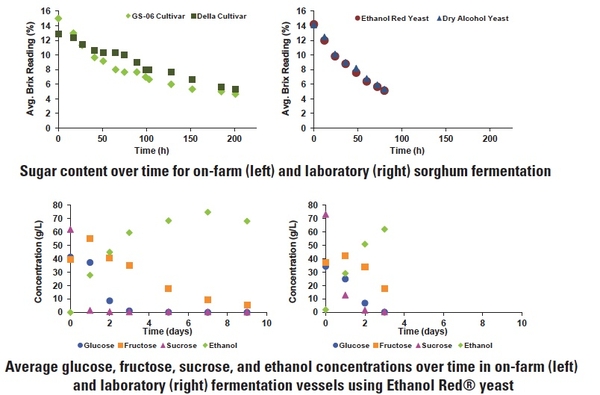
Results of our studies have shown that fermentation can be completed in 48 – 72 hours with 90% of sugars converted to ethanol simply by adding yeast to freshly pressed juice if proper conditions are maintained (Figure 6). Fermentation could occur faster if the temperature of the fermentation vessel is controlled and not left to ambient conditions. Determining the “recipe” for fermentation is difficult as variations in fermentation vessel size, yeast strain and starting concentration, environmental controls, and in some cases the variety of sweet sorghum used will influence aspects of the process.
In general, sufficient space should be provided in the fermentation vessel to allow for gas exchange. In other words, do not fill the fermentation vessel to the brim with the sweet sorghum juice. Leave 20% of the container empty to allow space for carbon dioxide generation. Also, yeast strains vary in performance.
Generally, supplementing with additional yeast will speed up the fermentation, but it will cost more. A low-cost option is to use the minimum amount of yeast needed and allow the yeasts to multiply to consume the sugar. This option will take longer, but it is cheaper than heavily loading the sugar solution with yeasts and potentially forcing production of alternative products.
Finally, be aware that allowing a fermentation to go on longer than 72 hours is detrimental to the process as other bacteria can begin feeding on the yeast and fermentation products, especially if vessel sterilization methods did not achieve a full kill and if the juice was not sterilized. Allowing a fermentation to continue unnecessarily and exposure to oxygen can also lead to the production of acetic acid (i.e., vinegar), which is worthless as a motor fuel. Typically the end of the fermentation is indicated by a reduced change or no change in sugar (Brix) content, and vessels contain a mixture composed primarily of yeasts, water, ethanol, and some residual fructose. Brix content and pH should be monitored periodically during fermentation to ensure the process is proceeding appropriately.
Distillation
Following fermentation, the vessel will contain a mixture of solids, ethanol, and water. Filtering the solution and concentrating the alcohol to above 190 proof is critical for proper operation in an engine. As the material exits the fermentation vessel, it is important to again filter the solution with mesh screens to remove the yeast and other solids. At this point, the solution can be distilled to remove water and produce a more pure form of alcohol.
Distillation is a process of separating various compounds based on differences in their boiling points. From an ethanol production standpoint, water and ethanol must be separated following fermentation. At sea level, ethanol has a boiling point of 173°F and water boils at 212°F. If the water-ethanol mixture is heated to a temperature between 173° and 212°F, the ethanol will turn into a gas and the water will remain a liquid. The ethanol gas will evaporate, thereby separating itself from the water. The ethanol vapors are then captured, cooled, and condensed back into a liquid. This process removes upwards of 90% of the water in the ethanol-water mixture; however, additional filtration is required to remove even more water if the ethanol will be used as a motor fuel. Generally, molecular sieves can be used to remove additional water, but this technology is too expensive and complex to implement at the farm level.
Several designs for distillation columns are available online or can be purchased from various companies that support home-brewing. It is very important, however, to check the laws and regulations in your locality to make sure ethanol distillation is legal.
Legality
Federal and state agencies do not distinguish between the manufacture of alcohol for human consumption and ethanol for fuel. This means ethanol production requires permitting from various agencies that govern the production of alcoholic beverages. Also, pure ethanol, like drinking alcohol, is subject to heavy excise taxes. The consumer should be weary of buying homemade ethanol unless it has been tested and meets the American Society of Testing and Materials (ASTM) specifications for fuel-grade ethanol, both from fuel quality and legal standpoints.
Ethanol is also known as ethyl alcohol, which is the same chemical compound found in many alcoholic beverages. The same basic fermentation and distillation processes are needed to create both fuel and beverage alcohol. The production process does vary slightly, however, depending on the starting material and final intended use. Fuel alcohol is denatured to prevent human consumption. Denaturing usually involves the addition of a small percentage of unleaded fuel to the ethanol product. This also circumvents the need for an excise tax.
Distillation is the primary operation monitored and controlled by state and federal governments. To insure you are in compliance with all laws, contact the state alcohol beverage commission (i.e., the ABC Board in North Carolina) and the Bureau of Alcohol, Tobacco, and Firearms (ATF). At a minimum, anyone who distills alcohol for fuel must complete forms for registration as an alcohol distillation operation that will need approval from law enforcement agencies.
References and Resources
The following materials were used in the development of this publication and can be referenced to acquire additional knowledge about using sweet sorghum in ethanol production.
Bennett, A.S., and R.P. Anex. 2008. Farm-gate production costs of sweet sorghum as a bioethanol feedstock. Trans. ASABE 51(2): 603-613.
Bridgers, E.N., M.S. Chinn, M.W. Veal, and L.F. Stikeleather. 2011. Influence of juice preparations on the fermentability of sweet sorghum. Biological Engineering, 4(2), 57-67.
Gnansounou, E., A. Dauriat, and C.E. Wyman. 2005. Refining sweet sorghum to ethanol and sugar: economic trade-offs in the context of North China. Bioresource Technology, 96(9), 985-1002.
Hunter, E.L., and I. C. Anderson. 1997. Sweet sorghum. In J. Janick (ed.), Horticultural Reviews, 21, pp. 73-104. New York, NY: John Wiley and Sons.
Kundiyana, D.K. 2006. “Sorganol”: In-field Production of Ethanol from Sweet Sorghum (doctoral dissertation).Stillwater, OK: Oklahoma State University.
Martini, A., P. Buzzini, and A. Vaughan-Martini. 2006. A microbiological perspective on renewable energy sources: Part 1. An overview on fermentation processes for the production of bioethanol from sweet sorghum. Chimica Oggi 24(1): 48-50.
Nan, L., and J. Ma. 1989. Research on sweet sorghum and its synthetic applications. Biomass 20(1-2): 129-139.
Nathan, R.A. 1978. Fuels from sugar crops: Systems study for sugarcane, sweet sorghum, and sugar beets. Washington, DC: Technical Information Center, U.S. Dept. of Energy.
Rains, G.C., J.S. Cundiff, and D.H. Vaughan. 1990. Development of a whole-stalk sweet sorghum harvester. Trans. ASAE 33(1): 56-62.
Smith, G.A., M.O. Bagby, R.T. Lewellan, D.L. Doney, P.H. Moore, F.J. Hills, L.G. Campbell, G.J. Hogaboam, G.E. Coe, and K. Freeman. 1987. Evaluation of sweet sorghum for fermentable sugar production potential. Crop Sci. 27(4): 788-793.
University of Georgia (UGA) Extension. 1999. Growing Sweet Sorghum for Syrup [Internet, Accessed Nov 2013). Athens, GA: UGA College of Agricultural & Environmental Sciences, Cooperative Extension Service.
Worley, J.W., D.H. Vaughan, and J.S. Cundiff. 1992. Energy analysis of ethanol production from sweet sorghum. Bioresour. Tech. 40(3): 263-273.
Acknowledgment
The development of this publication is based upon work supported in part by the BiofuelsCenter of North Carolina and the NC State University Extension Seed Grant program. The authors also acknowledge the assistance of Nicole Bridgers and Billy Duveray in carrying out field fermentation experiments.
Prepared by
Matthew W. Veal, Assistant Professor and Extension Specialist
Mari S. Chinn, Associate Professosr
Larry F. Stikeleather, Professor Matthew B. Whitfield, Graduate Research Assistant
Department of Biological and Agricultural Engineering
NC State University
Photographs provided by M. W. Veal
Publication date: Sept. 1, 2014
Reviewed/Revised: May 23, 2024
AG-786
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.