Overview
Currently available estimates place organic flue-cured tobacco production in North Carolina at 2,680 acres on 61 farms (Table 6-1). The farm gate value of this production system is estimated to be over $19 million (Table 6-1), which accounts for roughly 4.5% of the total value of tobacco in the state. As with conventional tobacco production, North Carolina is the leading U.S. producer of organic leaf (Table 6-1). Many questions remain regarding greenhouse management, organic nitrogen (N) source selection, field applications of approved organic N, and pest management. The information presented here is a summary of the organic tobacco research conducted by the Tobacco Research and Extension Programs at North Carolina State University.
| State | Farms | Acres |
Total Volume (lb) |
Total Value ($/state) |
Avg.Price ($/lb) |
Avg. Yield (lb/acre) |
|---|---|---|---|---|---|---|
| NC | 61 | 2,680 | 5,469,553 | 19,024,762 | 3.48 | 2,041 |
| PA | 3 | 6 | 17,400 | 59,400 | 3.41 | 2,900 |
| SC | 8 | 249 | 430,538 | 1,009,771 | 2.35 | 1,729 |
| VA | 26 | 1,378 | 3,017,830 | 10,844,094 | 3.59 | 2,190 |
| U.S. [b] | 105 | 4,396 | 9,140,079 | 31,644,057 | 3.46 | 2,079 |
[a] Table adapted from USDA-NASS, 2021. ↲
[b] U.S. total also includes Kentucky, Missouri, New York, and Tennessee. ↲
Greenhouse Management
Tobacco is a transplanted crop. As such, seedlings are produced in float systems contained within specialized greenhouses. In a tobacco float system, trays made of expanded polystyrene (Styrofoam) or plastic are filled with a peat moss-based soilless media complex that contains trace amounts of agricultural lime and fertilizer. Individual tobacco float trays measure approximately 13 x 26 inches and contain 288 to 338 individual cells, depending upon grower preference. Each cell is filled with soilless media, dibbled with a roll bar, and a single pelletized tobacco seed is placed in the center of each dibble. The trace quantity of fertilizer within the media blend is not enough to promote sufficient seedling growth and development. Therefore, supplemental nutrients must be added to the underlying water solution 7 to 10 days after floating and again roughly three weeks later. Small openings on the underside of each cell in a float tray allow the fertilized water source to wick upwards to the root zone of seedlings. It takes 50 to 60 days for seedlings to reach a size that is appropriate for transplanting, during which time the seedlings should be clipped 1.5 inches above the bud at least five times (but can be more) to promote seedling uniformity and hardiness.
In conventional production systems, producers commonly use complete, water-soluble fertilizer sources having either a 2-1-2 or 3-1-3 fertilizer ratio, as well as appropriate quantities of secondary and micro-nutrients required by tobacco seedlings. These fertilizer sources are prohibited from use in the production of certified organic transplants. Organic producers must use OMRI-listed sources that are approved by third-party organic certifiers as well as the purchasers of organic leaf. Many of these OMRI-listed products are agricultural animal byproducts, such as feather meal, poultry litter, and guano. Unlike conventional fertilizers, which contain plant-available nutrients, these organic products require the actions of microbes to break down the complex organic molecules into a form that can be readily used by plants. At present, most producers rely heavily upon Peruvian seabird guano as the primary source of nutrients in organic greenhouse systems.
Peruvian seabird guano (SG) is a natural product mined from seabird guano deposits commonly found off the coast of South America. Various formulations and brands of SG exist; however, those most commonly used by tobacco producers are high in organic nitrogen (N) and phosphorus (P) and low in potassium (K) (such as Sunleaves® 12-11-2). Many producers have expressed concern with the use of fertilizer sources high in organic nitrogen due to the negative effects the source can have on seedling development, specifically as urea is released from the nutrient source. In addition, as producers add SG to the float water at rates designed to supply sufficient nitrogen (typically between 125 and 150 ppm N), they often over-supply phosphorus (3x) and under-supply potassium (-6x). Additionally, tobacco float beds fertilized with SG often contain extremely high concentrations of bicarbonate (HCO3-), which can increase water pH, limit nutrient availability, and reduce seedling growth and vigor. In addition to a range of 125 to 150 ppm N, an ideal float bed fertility program will provide 35 to 50 ppm P and 125 to 150 ppm K.
To improve nutrient recommendations for organic tobacco producers, research was conducted to evaluate three organic nitrogen (N) programs that might serve to address the following:
- Provide sufficient N for seedling growth.
- Limit phosphorus exposure.
- Reduce bicarbonate concentrations (prevent high float water ph).
The three organic N programs evaluated were 100% SG, 100% sodium nitrate (SN; SQM Allganic® 16-0-0), and SG + SN. Sodium nitrate is a mined compound from South America that is OMRI-listed and contains 100% nitrate-N. Treatments were supplemented with OMRI-listed water soluble 0-0-52 (potassium sulfate, SQM Allganic®). Each fertility program was designed to provide 125 ppm N, between 0 and 115 ppm P, and 125 ppm K. Three additional treatments of each organic N program that included gypsum (calcium sulfate) were also evaluated. Each treatment was compared to a conventional, homogenized water-soluble fertilizer source (SQM 16-5-16). A complete list of treatments and the nutrients supplied by each fertilizer program can be found in Table 6-2.
After seeding, float water solution samples were collected every five days until the conclusion of the study. Prior to float water collection, water temperature and dissolved oxygen content were measured with a Dissolved Oxygen meter. Fertilizer sources were added to each floatbed at nine and 21 days after seeding (floating), with 50% of the total fertilizer treatment being applied at each interval. Prior to fertilizer addition, each source was combined (Table 6-2) into a 16-fl-oz bottle and dissolved into hot water (122°F). Seabird guano was ground to pass through a 1-mm sieve in order to increase solubility. Total seedlings, usable seedlings, seedling stem height, and seedling stem diameter were measured and recorded at the conclusion of the study.
| Fertilizer Program [a] | N (ppm) | P (ppm) | K (ppm) | Ca (ppm) | Quantity (oz/100 gal water) |
|---|---|---|---|---|---|
| 16-5-16 | 125 | 40 | 125 | 0 | 10.4 |
| SG + PS | 125 | 115 | 20(SG) + 105(PS) | 0 | 13.9 + 2.7 |
| SN + PS | 125 | 0 | 125 (PS) | 0 | 10.4 + 3.2 |
| SG + SN + PS | 44(SG) + 81(SN) | 40 | 7(SG) + 118 (PS) | 0 | 4.9 + 6.8 + 3.0 |
| SG + PS + Gyp | 125 | 115 | 20(SG) + 105(PS) | 50 | 13.9 + 2.7 |
| SN + PS + Gyp | 125 | 0 | 125(PS) | 50 | 10.4 + 3.2 |
| SG + SN + PS + Gyp | 44(SG) + 81(SN) | 40 | 7(SG) + 118 (PS) | 50 | 4.9 + 6.8 + 3.0 |
[a] SG, Seabird Guano; PS, potassium sulfate; SN, sodium nitrate; Gyp, gypsum. ↲
Results
Treatments containing SN as the sole source of nitrogen failed to produce usable seedlings due to the absence of phosphorus in the selected fertilizer program (Table 6-3) and the low phosphorus (<1.0 ppm) content of the soilless media source. Seedling growth and development was acceptable in treatments of SG or SG+SN, and was similar to that of 16-5-16 (Table 6-3). In addition, it does not appear that calcium was a limiting production factor; therefore, gypsum was not required for plants to reach optimum transplanting size. It is probable, however, that calcium demand might vary from season to season, based upon growing conditions. Should calcium deficiency develop, producers are encouraged to utilize OMRI-listed sources of gypsum. The use of lime is discouraged, as it may increase the solution pH to a level that limits the availability of other nutrients—in much the same way as bicarbonates.
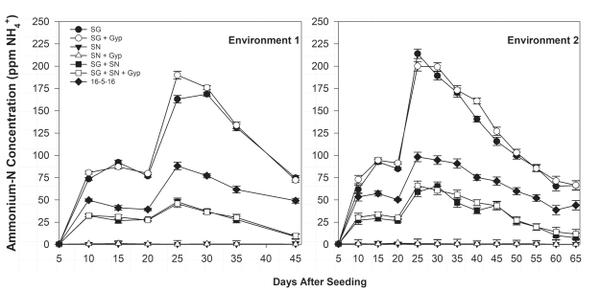
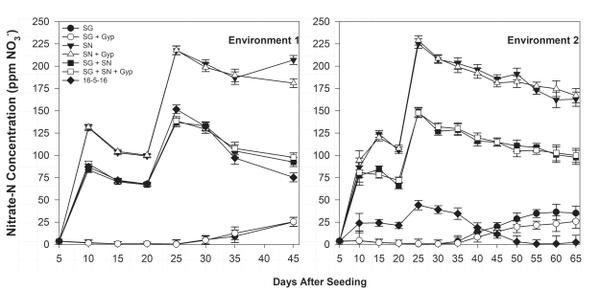
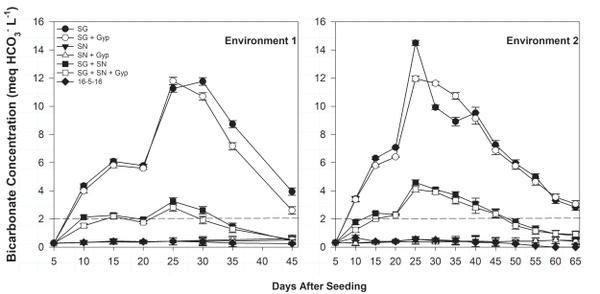
Ammonium-N float water concentration was greatest in SG treatments 25 days after seeding (DAS) but declined rapidly over the following 20 to 30 days (Figure 6-1). The decline in ammonium concentration was complemented by an increase in nitrate-N concentration during the same period (Figure 6-2), indicating ammonium conversion to nitrate. Bicarbonate concentration was greatest in SG only (≥12.0 meq/L) and SG+SN (≥3.0 meq/L) treatments 25 DAS but was <1.0 meq/L in SN-only treatments, further implicating SG as a source of bicarbonates in organic float systems (Figure 6-3). The established bicarbonate limit is 2.0 meq/L, beyond which acidification is recommended. Contact your local Extension agent for rates of organic acidifiers. Despite the high bicarbonate concentrations documented in SG treatments, seedling growth was not impacted. Ultimately, SG- and SG+SN based fertility programs produced seedlings comparable to 16-5-16 and appear to be suitable for the production of organic tobacco seedlings (Table 6-3). These fertility programs should be managed to include additional nutrients, such as phosphorus, in order to provide a complete nutrition program. Furthermore, bicarbonates should be monitored and corrected accordingly. While it did not appear to cause any deleterious effects, dissolved oxygen values in the SG and SG+SN treatments dropped precipitously after the final fertilizer application. This drop is most likely caused by high rates of respiration in the aerobic bacterial populations in the float system solution. Placement of an airstone or other aeration device within the float system solution should ameliorate this issue and may aid in increasing the rate of nitrification.
| Fertilizer Program [b] | Total Plants (%) | Usable Plants (%) | Stem Diameter (mm) | Stem Height (cm) |
|---|---|---|---|---|
| 16-5-16 | 88 a | 79 bc | 3.30 a | 6.62 a |
| SG + PS | 90 a | 78 c | 2.76 b | 5.62 b |
| SN + PS | [c] | |||
| SG + SN + PS | 91 a | 85 ab | 3.48 a | 6.27 ab |
| SG + PS + Gyp | 91 a | 79 bc | 2.84 b | 6.08 ab |
| SN + PS + Gyp | ||||
| SG + SN + PS +Gyp | 93 a | 86 a | 3.43 a | 6.47 ab |
[a] Treatment means followed by the same letter within the same column are not significantly different. ↲
[b] SG = Seabird Guano; PS = potassium sulfate; SN = sodium nitrate; Gyp = gypsum. ↲
[c] Treatment did not produce usable transplants; therefore, data are excluded from the analysis. ↲
Additional Points for Consideration
- Producers might consider processing (grinding and dissolving) SG prior to application. Smaller SG particles will have a higher surface area and will be more water soluble, both of which should increase nitrogen release.
- When blending nitrogen sources, target 40 ppm phosphorus from SG (4.85 oz. 12-11-2/100 gallons float water). This will ensure phosphorus for the season when added to the float water in two separate applications.
- Consult with your local Extension Agent if you suspect a deficiency (such as calcium or boron). Organically approved secondary and micronutrient sources are available but need to be confirmed.
- Consult with your organic certifier prior to the use of ANY fertilizer source.
- Water circulation is critical for organic nutrient sources, as some (such as guano) are not easily dissolved. Submersible pumps will help circulate water and nutrients and can add oxygen to the float water. The addition of oxygen is recommended as it will help promote nitrification.
- 2.90 oz gypsum/100 gallons of float water will add roughly 50 ppm calcium and 40 ppm sulfur.
- Float water samples should be collected and analyzed at frequent intervals.
Field Production Management of Nitrogen
In conventional production systems, N sources that contain a large portion of mineral-N (nitrate, ammonium, or a combination of both) are preferred to sources high in organic-N. The most commonly used N sources in conventional systems are calcium nitrate (100% nitrate-N), potassium nitrate (100% nitrate-N), ammonium nitrate (50% nitrate-N + 50% ammonium-N), liquid urea-ammonium nitrate (25% nitrate-N + 75% ammonium-N), and ammonium sulfate (100% ammonium-N). The preferred nitrogen source of tobacco is nitrate-N, and in the Southeastern United States the ammonium-N contained in some of these sources is rapidly converted into nitrate-N (typically between 3 and 14 days).
Conversion of ammonium-N to nitrate-N is promoted by moderate soil pH (5.8 to 6.2 for tobacco soils), warm soil temperature, and moderate soil moisture. Conventional, synthetic N sources are prohibited from use in organic systems. Sodium nitrate (100% nitrate-N) can be used in organic systems, though it can account for no more than 20% of the total N applied per acre in a given season. For this reason, producers are required by USDA-NOP and tobacco industry standards to utilize organic N sources as opposed to synthetic or mined sources.
Given that organic-N sources of commercial fertilizer are often very low in mineral-N at the time of application, and that soil moisture can be limiting during certain portions of the growing season, N sources used in the production of organic tobacco must be given careful consideration prior to use. One organic N source that offers great promise is hydrolyzed poultry feather meal. Hydrolyzed poultry feather meal (PFM) is a high-nitrogen byproduct (≈15% N; Hadas and Kautsky, 1994; Hartz and Johnstone, 2006) of the livestock processing industry. Poultry feather meal might serve as a quick-release N source with limited late-season availability.
To evaluate PFM, research was recently conducted to quantify the effects of two PFM sources (Nature Safe® 13-0-0 and NutriMax® 12-1-0) applied at three rates on the yield, quality, and chemical constituents of flue-cured tobacco. The three application rates were 15 pounds N below base recommendation (-B), at base recommendation (B), and 15 pounds above base recommendation (+B). Studies were conducted at the Lower Coastal Plain Research Station in Kinston, North Carolina, and the Oxford Tobacco Research Station in Oxford, North Carolina, in both years, accounting for a total of four different growing environments. Base application rates were 70 and 75 pounds of N per acre in Kinston and Oxford, respectively. All nitrogen was broadcast-applied prior to transplanting and incorporated with a disc.
Results from this study indicate that both Nature Safe® and Nutrimax® fertilizer sources are acceptable for producing flue-cured tobacco in organic systems. Both sources appear to fit the needs of high-nitrogen materials, with rapid early-season N release, and sharp declines in availability as the season progresses. The lack of visual late-season greening likely indicates that N release from both sources occurred in the early portion of each growing season. Application rates of either organic N source evaluated should follow the recommendations provided by NC State Extension, which are specific to individual growing environments. While we did not observe delayed N mineralization and assimilation in the two years of our study, we remain concerned about this issue under adverse or dry growing conditions. These concerns are amplified where application rates are excessive, and precipitation is below average or occurs at irregular intervals throughout the growing season. Conversely, producers should not reduce N application rates to hedge against greening related issues. Generally, the lower N application rates evaluated in this study lowered total alkaloid (nicotine) concentration and increased the reducing sugar concentration (Table 6-4). While this factor did not have great agronomic significance, it could have much larger implications to smoke flavor and aroma, two factors not evaluated in this study.
Additional studies have also been conducted to determine the most appropriate application timings for organic N sources. In these studies, two organic N sources (Nature Safe® 13-0-0 and NutriMax® 12-1-0) were applied 100% broadcast prior to transplanting (BC), 50% broadcast prior to transplanting + 50% sidedress 10 days after transplanting (BC/SD), or 100% sidedress 10 days after transplanting (SD; Table 6-5). Performance of Nature Safe® and Nutrimax® were similar. In addition, each of the three application methods produced similar results in a season that was characterized by relatively normal environmental conditions. Alternatively, in a different season, the 100% SD application program generally increased leaf nitrogen concentration and cured leaf yield. This growing season was marked with excessive, season-long precipitation. The delay of N application 10 days after transplanting may have reduced the potential for leaching. In addition, the placement of nitrogen in such close proximity to the rooting zone would have increased fertilizer-use efficiency, thus overcoming severe rainfall to some degree. Cured leaf quality was not influenced by nutrient application. Ultimately, each of the three application methods is likely acceptable for tobacco production as rainfall patterns are unpredictable at the onset of any growing season. Producers are encouraged to consider each application program based upon equipment capabilities and material availability. Regardless, producers are discouraged from making organic N applications at layby or later in the season.
Additional sources of nitrogen have been evaluated for field application in tobacco. At present, these sources are not commercially available, cost prohibitive, or difficult to apply in a manner that is consistent with the recommendations previously outlined. These products are blood meal, corn gluten, and soy protein. Composted layer manure has also been tested, but produced tobacco that was lower in yield, lighter in color, and with a different chemistry than that which was treated with feather meal. It is hypothesized that the mineralization of composted layer manure may be slower than that of feather meal, leading to less N assimilation during the growing season.
| Effect | Yield (lb/a) | QI [b] | SPAD | Layby (% N) | Topping (% N) | Cured (% N) | TA [e]
(%) |
RS [f] (%) |
|
|---|---|---|---|---|---|---|---|---|---|
| Source | NS [c] | 2,458 a | 84 a | 42.5 a | 4.25 a | 2.73 a | 1.65 a | 2.49 a | 17.6 a |
| NM [d] | 2,361 a | 82 b | 42.1 a | 4.23 a | 2.64 a | 1.65 a | 2.47 a | 17.7 a | |
| Rate | -B | 2,385 a | 83 a | 42.0 a | 4.14 b | 2.63 a | 1.62 a | 2.36 b | 17.8 ab |
| B | 2,387 a | 83 a | 41.7 a | 4.20 ab | 2.68 a | 1.65 a | 2.49 a | 17.9 a | |
| +B | 2,455 a | 83 a | 43.3 a | 4.38 a | 2.74 a | 1.68 a | 2.59 a | 17.2 b |
[a] Treatment means followed by the same letter within the same column and main effect are not significantly different. ↲
[b] QI, quality index-assessed on a scale of 1 to 100, with 100 having the highest quality. ↲
[c] NS, Nature Safe® (13-0-0). ↲
[d] NM, NutriMax® (12-1-0). ↲
[e] TA, total alkaloids. ↲
[f] RS, reducing sugars. ↲
| Application Method | Cured Leaf Yield (lb/acre) | |||
|---|---|---|---|---|
| Kinston 2012 | Oxford 2012 | Kinston 2013 | Oxford 2013 | |
| Broadcast | 2,306 a | 2,564 a | 1,675 b | 3,083 b |
| Broadcast + Sidedress | 2,370 a | 2,604 a | 1,764 ab | 2,860 b |
| Sidedress | 2,174 a | 2,425 a | 1,925 a | 3,389 a |
[a] Treatment means followed by the same letter within the same column and main effect are not significantly different. ↲
Management of Other Plant Essential Nutrients
Phosphorus (P)
As is the case in conventional production systems, adequate supply of P is typically found in most North Carolina soils that have a history of tobacco production. Routine soil testing will aid in determining if in-season application is needed. If the nutrient is required, some fertilizer sources approved for organic production, such as Nature Safe® 8-5-5, contain sufficient amounts of P. However, producers should only apply enough fertilizer to satisfy P demand as reflected in a soil testing report. This might limit the quantity of applied nitrogen but the difference can be made up through the application of organic fertilizers absent of P. Producers should also be made aware that these fertilizer sources are not easily dissolved in water; therefore, transplant water applications are discouraged. At present, commercial transplant water fertilizers are not available for organic systems.
Potassium (K), Sulfur (S), and Magnesium (Mg)
Targeted K application rates should reflect those used in conventional systems. Organic K sources are typically composed of sulfate of potash or sulfate of potash magnesium (Sul-Po-Mag) and do not require mineralization upon application. Potassium fertilizers can be applied pre-bedding or post-transplanting depending upon grower preference. Application rates of K fertilizer sources should also provide adequate quantities of S. Magnesium supply should likewise be suitable when Sul-Po-Mag is used or when dolomitic limestone is used as a liming material for pH adjustment. Producers should verify that these fertilizer sources are approved for organic production prior to application.
Sucker Control
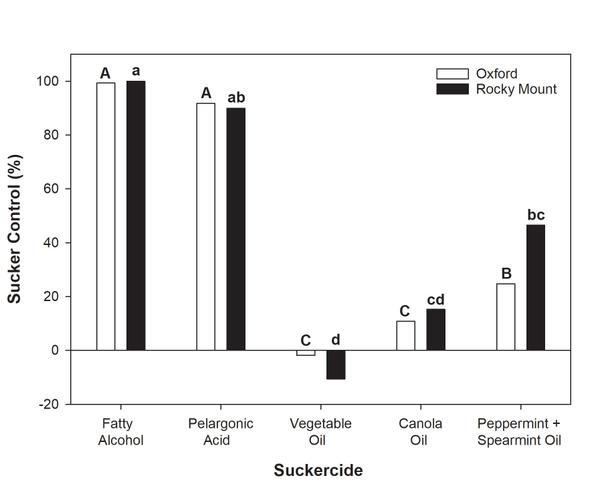
Sucker growth after topping is a management issue that producers must address in organic tobacco production. In recent years, commercial producers have been able to apply organically approved formulations of fatty alcohol products (such as O-Tac and Green-Tac). The application rates and patterns of these suckercides should follow practices that have been historically utilized in conventional production systems. For example, the first fatty alcohol application should be made when 50% of the plants in a field are in the elongated button stage of growth, with repeated applications every five to seven days thereafter. Due to the absence of systemic suckercides with residual control in organic systems, producers are encouraged to make as many applications as they are comfortable with. Reports from growers suggest that the number of fatty alcohol applications will vary from season to season, but may range from a minimum of four to as many as eight. Regardless, the first application should contain a 4% solution concentration (2.0 gallons of product + 48 gallons of water) with subsequent application delivery occurring at a 5% concentration (2.5 gallons of product + 47.5 gallons of water). Application programs consisting of this program contained in six applications provided 98 to 100% control in trials conducted in North Carolina in 2018 (Figure 6-4). *Treatment means followed by the same lowercase or uppercase letter are not significantly different at the ɑ=0.05 level. Despite the sufficient control offered by fatty alcohol materials, producers should remember that suckers larger than one inch in length will not be sufficiently controlled by chemical application, and that hand-suckering should be utilized before any application, where necessary.
Alternatives to fatty alcohol products have also been evaluated for use in organic tobacco production. At present, these alternatives have proven to either have limited efficacy or produce unacceptable leaf injury following application. For example, pelargonic acid application may provide ≥85% sucker control but is likely to produce unacceptable levels of leaf injury. In a recent trial, injury following pelargonic acid application ranged from 32% to 72%, while that in fatty alcohol treatments was <3%. Other oil-based products (such as vegetable oil and canola oil) have also been evaluated, but have very limited efficacy (Figure 6-4) when diluted using the high water volumes currently associated with chemical sucker control (50 gallons per acre).
Insect Management
For specific information on registered pesticides, recommended use rates, and use patterns, refer to NC State’s North Carolina Agricultural Chemicals Manual (AG-1). Acceptable (OMRI-listed) organic materials recommended for use in tobacco are indicated in “Precautions & Remarks.” The information presented here and in the Agricultural Chemicals Manual is done so in good faith and was accurate at the time of publication. It is, however, subject to change. When using pesticides, always follow the instructions on the product label. The label is the law!
More detailed information on insect pest biology and suggested treatment thresholds is presented in the Flue-Cured Tobacco Guide (AG-187) and NC State’s Tobacco Insect Management page.
Greenhouse Pest Management
Insects in tobacco production greenhouses are typically opportunistic pests and can be minimized through good sanitation and production practices. Greenhouses should be used only for tobacco production, tobacco seedlings should be removed promptly after transplanting is complete, and weeds should be prevented. Avoid storing materials in greenhouses, and remove trash to minimize places pests can harbor during winter and summer.
Slugs and fire ants can occasionally damage seedlings. There are effective, organically acceptable baits for both of these potential pests.
Aphid and caterpillar pests may occasionally infest greenhouses when pre-transplant conditions are warm. Materials containing Bacillus thuringiensis (Bt) are effective against caterpillars and can typically be used in greenhouses. There are few effective pesticides for use against aphids in tobacco in the field or in the greenhouse. Caution should be taken when considering the use of EC formulations or oils on plants in the greenhouse as they can cause phytotoxicity.
Soil Insect Management
Soil insect pests of tobacco include wireworms, white fringed beetles, vegetable weevils, and sod webworms. Field selection and crop rotation are the most effective organic management strategies for soil insect pests. Fields that are being converted from pasture or fallow land and those in rotations following small grains and corn may have higher soil insect populations. Fields in rotation following sweetpotato or soybean are less likely to have damaging soil insect populations. If a field has a history of wireworms or nematodes, sweetpotato is not recommended for rotation. Producers should consider both insect pressure and disease history of fields when selecting their rotation.
Field Insect Pests
Cutworms
A complex of cutworm species (including variegated cutworm, black cutworm, and spotted cutworm) can damage tobacco in the month following transplant. Damaging populations of cutworms are rare but may be higher in small fields near wooded areas or fields surrounded by weedy vegetation. Scout fields weekly for cutworm damage during the first four weeks after transplant. Cutworms first produce small feeding holes in leaves and then later begin their characteristic “clipping” of plants. Cutworms are nocturnal, so return to damaged spots in the evening to check for larvae. If the number of clipped plants is approaching 10%, management is recommended. Materials containing Bt are effective for use against cutworms.
Tobacco Flea Beetles
Tobacco flea beetles may damage plants for the first several weeks post-transplant and occasionally again near harvest. Tobacco flea beetles are small (less than 1/16 of an inch long) and create shot-hole-like injury to leaves. Post-transplant, the treatment threshold for tobacco flea beetles is four per plant. Of the currently labeled insecticides approved for organic production, Pyganic is likely the most effective (Table 6-6).
Green Peach Aphids
Aphids are the most difficult-to-control foliar pest in organic tobacco production. There are few effective insecticides (Table 6-6). Aphid populations typically develop five to eight weeks post-transplant but do not persist post-topping as plants mature. Acceptable organic contact sucker control materials can also kill aphids through direct contact. Therefore, the most effective aphid management strategy is timely applications of sucker control materials and topping.
Tobacco Budworm
Tobacco budworms feed on buds and flowers pre-topping. Post-topping, they are no longer a concern, because these parts of the plant have been removed. They can occasionally infest suckers and then feed on harvestable leaf, but good sucker control eliminates this risk. Parasitoid wasps and predators can kill up to 90% of the budworm larvae in a field, and budworm feeding injury rarely results in yield loss as plants can compensate for lost leaf tissue post-topping. However, if budworm populations reach concerning levels, baits containing Bt are generally the most effective control options. Sprayable Bt materials are typically less effective against tobacco budworm because achieving spray coverage into the bud can be difficult.
Tobacco and Tomato Hornworms
Hornworms are most common post-topping in tobacco but can occasionally occur pre-topping. Numerous natural enemies attack hornworms in tobacco, including predatory bugs, wasps, parasitic wasps, and parasitic flies. The current recommended treatment threshold for hornworms in tobacco is one per 10 plants, and caterpillars with white parasitic wasp cocoons on their backs are counted as ⅕ of a worm. This is because these larvae eat one-fifth as much as non-parasitized larvae. Bt is very effective against hornworms, and materials containing neem may also provide some level of control.
Enhancing Biological Control in Tobacco
Planting flowering plants such as sunflower, buckwheat, or clover near or in strips within tobacco fields has been recommended as a strategy to increase the local abundance of predatory and parasitic insects, particularly lady beetles and parasitoid wasps, which attack aphids. We have investigated the effect of sunflower and buckwheat plantings on beneficial and pest insect populations in tobacco and have found very small increases in predatory insects and very small decreases in some pest insects. These effects are so small, however, that we do not believe they are likely to provide sufficient pest control to offset their cost. In addition, sunflower is a host to bacterial wilt. Sunflower planted in fields with a history of bacterial wilt can harbor and increase populations of the bacterium, increasing disease pressure.
Augmentative biological control, specifically the release of lady beetles, has also been recommended as a method to control aphids in tobacco. Numerous experiments in many different crops have attempted to determine if lady beetle releases can be used to control pests in the field and none have found them to be effective. This is not a recommended management strategy in tobacco.
References
Hadas, A. and L. Kautsky. 1994. “Feather Meal, A Semi-Slow-Release Nitrogen Fertilizer for Organic Farming.” Fertilizer Research 38: 165–170.
Hartz, T.K. and P.R. Johnstone. 2006. “Nitrogen Availability From High-Nitrogen-Containing Organic Fertilizers.” HortTechnology 16 (1): 39–42.
USDA-NASS. 2017. Certified Organic Survey-2016 Summary (September 2017). Surveys–Certified Organic Survey. United States Department of Agriculture-National Agricultural Statistics Service. (accessed December 13, 2023).
Disease Management
An effective organic disease management program integrates a combination of cultural practices and chemical applications. Organic chemistries are often less mobile and are non-systemic, which means that relying on them solely will result in losses. It is also important to develop a disease management strategy before the crop is planted, such that measures can be taken before diseases reach an economic threshold. To select appropriate tools, accurate identification of perennial disease pressures is important. Many cultural practices are inherently organic and are designed to reduce inoculum survival either through crop rotation, crop debris destruction, or selection of a disease-resistant variety.
Crop Rotation
Most economically significant diseases affecting tobacco are caused by pathogens that persist in the soil and can only reproduce in a limited range of hosts. In the absence of tobacco or other host plants, the soilborne pathogens start to die and the populations (propagules) are reduced. Therefore, crop rotation must be emphasized in planning any disease management program. Not only does crop rotation benefit tobacco, but it also improves the sustainability and yield potential of other valuable crops. There are certainly several regions in North Carolina that have few rotational crop selections due to environmental conditions; however, emerging crops may also provide a unique rotational benefit to tobacco producers for disease management. Many commonly grown North Carolina crops are good in rotations to help control tobacco diseases (Table 6-7).
| Crop | Black Shank | Black Root Rot | Granville Wilt | Tobacco Mosaic Virus | Root-Knot |
|---|---|---|---|---|---|
| Corn | High | High | Mod. | High | Low |
| Cotton | High | Low | Mod. | High | None |
| Fescue | High | High | High | High | High |
| Lespedeza “Rowan” | High | Low | High | High | High |
| Milo | High | High | Mod. | High | Low |
| Peanuts | High | Low | Low | High | None |
| Pepper | High | High | None | None | None [a] |
| Potato, white | High | High | None | High | Low |
| Small grain | High | High | High | High | High |
| Soybean | High | Low | High | High | Low [b] |
| Sweetpotato | High | High | Mod. | High | Low [c] |
| Tomato | High | Mod. | None | None | None [b] |
Note: These ratings are based on the assumption that weeds are well-managed in these crops. Ratings range from high to none. High = highly valuable as a rotation crop for this disease; none = no value as a rotation crop, may be worse than continuous tobacco.
[a] Rating may be high for certain root-knot species or races. ↲
[b] Rating is high if a root-knot resistant variety of soybean or tomato is used. ↲
[c] Rating is moderate if a root-knot resistant variety of sweetpotato is used. ↲
Length of Rotation
When rotation is not implemented and tobacco is grown continuously, pathogen populations are allowed to build up and the risk of severe disease development increases. The longer the rotation, the greater the reduction of soilborne pathogen populations. A four-year rotation, in which three alternate crops are planted between tobacco, is more effective than a two- or three-year rotation. Similarly, a three-year rotation is more effective than a two-year rotation. Nevertheless, a two-year rotation, in which one alternate crop is planted between crops of tobacco, significantly reduces disease and is far more beneficial than continuous tobacco production.
Stalk and Root Destruction
Roots and stalks from the previous year’s crop must be destroyed, regardless of whether diseases have been observed in a given year (Table 6-8). To be effective, this must be accomplished as soon after harvest as possible. Completing these tasks reduces populations of several tobacco diseases, including black shank, Granville wilt, root-knot nematode, tobacco mosaic virus, brown spot, and tobacco vein banding mosaic virus, as well as certain insects, grasses, and weeds.
Furthermore, destroying old tissue exposes pests living in the tissues to adverse environmental elements. For example, root-knot nematodes are very sensitive to drying; if root tissue surrounding them decays, they are exposed to drying conditions. Tobacco mosaic virus (TMV) particles lose their ability to infect after they are freed from tobacco tissue. By destroying tobacco roots and stalks, TMV carryover may be reduced to a level at which less than 1% of tobacco plants become infected at transplanting. Removing TMV-infected tobacco plants before first cultivation may also prevent secondary spread.
Resistant Varieties
Growers should not depend solely on resistant varieties for disease management. Even resistant varieties are sometimes severely damaged by disease, especially where rotation, stalk and root destruction, and other management tools are not used. Some varieties are highly resistant to only certain races or species of a particular pathogen. For example, root-knot-resistant varieties are only resistant against Meloidogyne incognita, races 1 and 3. Some of the varieties listed in Table 6-9 are highly resistant to race 0 of the black shank pathogen but are quite susceptible to race 1. See the section on black shank for a more complete discussion of resistance to that disease, and Table 6-9.
| Variety [a] | Ph gene [b] | Black Shank | Granville Wilt | RKN [f] | TMV [g] | ||||
|---|---|---|---|---|---|---|---|---|---|
| % Survival [c] | Disease Index [d] | Resistance [e] | % Survival [c] | Disease Index [d] | Resistance [e] | ||||
| CC 13* | - | 57.43 | 22.74 | M | 41.95 | 24.78 | M | R | S |
| CC 27 | + | 38.86 | 37.18 | L | 48.83 | 29.76 | M | R | R |
| CC 33 | - | 71.32 | 14.87 | H | 40.67 | 32.99 | M | R | S |
| CC 35* | - | 81.71 | 34.26 | M | 12.36 | 41.84 | L | R | S |
| CC 37* | + | 39.08 | 24.10 | M | 66.87 | 19.83 | M | R | R |
| CC 67* | + | 59.51 | 23.51 | M | 53.08 | 26.18 | M | R | R |
| CC 143* | - | 55.58 | 6.94 | H | 46.07 | 28.91 | M | R | R |
| CC 144 | + | 84.34 | 2.69 | H | 54.17 | 13.66 | H | S | S |
| CC 145 | NA | 90.61 | 2.47 | H | 35.00 | 14.95 | H | S | S |
| CC 700* | + | 67.44 | 18.42 | M | 35.59 | 34.53 | M | R | S |
| CC 1063* | - | 91.88 | 3.24 | H | 53.13 | 27.33 | M | R | S |
| GF 318 * | + | 60.32 | 19.17 | M | 41.32 | 33.23 | M | R | R |
| GL 26H * | - | 53.22 | 16.10 | M | 41.79 | 26.09 | M | R | R |
| GL 365 * | NA | 93.73 | 2.03 | H | 78.26 | 6.82 | H | R | S |
| GL 395 * | - | 67.06 | 16.53 | H | 44.86 | 32.90 | M | R | S |
| GL 976 | + | 46.54 | 18.64 | M | 11.29 | 44.34 | L | R | R |
| K 326 * | - | 37.03 | 34.97 | L | 24.06 | 39.95 | L | R | S |
| K 346 * | - | 85.37 | 7.79 | H | 49.10 | 30.44 | M | R | S |
| NC 71 * | + | 43.52 | 32.49 | M | 27.27 | 39.42 | L | R | S |
| NC 72 * | + | 56.81 | 19.22 | M | 34.09 | 37.69 | L | R | S |
| NC 196 * | + | 72.95 | 14.80 | H | 39.86 | 38.32 | L | R | S |
| NC 297 * | + | 36.78 | 43.76 | L | 55.07 | 19.71 | M | R | R |
| NC 299 * | + | 46.57 | 26.89 | M | 46.05 | 29.64 | M | R | S |
| NC 606 * | - | 77.33 | 9.23 | H | 67.38 | 17.13 | H | R | S |
| NC 925 * | - | 92.38 | 3.75 | H | 41.98 | 34.64 | M | R | S |
| NC 938 * | - | 86.90 | 7.29 | H | 50.59 | 29.53 | M | R | S |
| NC 940 | - | 74.01 | 18.54 | M | 53.03 | 26.36 | M | R | S |
| NC 970 | + | 68.37 | 11.70 | H | 4.40 | 56.94 | L | R | S |
| NC 972 | + | 90.31 | 3.24 | H | 5.94 | 53.77 | L | R | S |
| NC 1226 | + | 98.55 | 0.52 | H | 12.25 | 40.68 | L | R | S |
| PVH 1452 * | + | 66.41 | 15.63 | H | 52.75 | 25.50 | M | R | S |
| PVH 1600 * | + | 64.34 | 21.22 | M | 47.47 | 29.02 | M | R | S |
| PVH 1920 * | + | 78.25 | 6.49 | H | 52.96 | 28.62 | M | R | S |
| PVH 2110 * | - | 49.69 | 26.96 | M | 35.26 | 38.34 | L | R | S |
| PVH 2254 * | - | 49.58 | 21.68 | M | 43.32 | 34.96 | M | R | S |
| PVH 2275 * | + | 7.31 | 58.57 | L | 48.11 | 30.66 | M | R | S |
| PVH 2310 * | + | 29.80 | 38.17 | L | 37.05 | 30.04 | M | R | S |
| PVH 2360 | NA | 24.78 | 29.49 | L | 66.67 | 18.35 | H | R | R |
[a] Variety names followed by a * indicate a commercially available variety ↲
[b] Varieties containing the Ph gene are labeled with +, those without the pH gene labeled with -. Varieties with no information available are labeled with NA. ↲
[c] Percentage of survival ratings are the percent survival based on the observed percent of healthy plants across three years of data collection in fields heavily infested with disease. High ratings equate to a higher level of resistance. ↲
[d] Disease Index ratings are the average disease index across three years of data and several growing regions heavily infested with disease. The lower the disease index rating, the higher the level of resistance in a given variety. ↲
[e] Resistance level designated as L= Low, M = Moderate, and H = High ↲
[f] Varieties with resistance to root-knot nematode = R, susceptible varieties = S ↲
[g] Varieties with resistance to Tobacco Mosaic Virus = R, susceptible varieties = S ↲
Additional Useful Practices
The following practices give the plant every possible advantage to withstand attack by disease-causing agents. Carefully considering the impact of each practice on disease development and operating in ways that favor tobacco plant development work to the disadvantage of disease-causing agents and improve management of diseases in the field.
Site Selection
Certified organic production sites have many restrictions, which can limit availability of high-yielding sites. Because of the limited availability of effective organic fungicides, selection of a site with low disease pressure will help reduce inputs required for organic production. Sites with a history of severe disease may not be favorable for generating high-yielding, high-quality tobacco.
Formation of a High, Wide Bed (Row)
Developing a high, wide bed in the field helps provide proper conditions for tobacco roots to develop. This practice conserves soil moisture during dry periods and helps provide drainage for root systems in areas of fields that tend to become waterlogged. Most causal agents that affect the root systems of plants are favored by poor drainage and high-moisture environments.
Spacing
Tobacco plants that are spaced too closely often suffer more disease than those planted farther apart in the row. In particular, spacing influences diseases such as brown spot, target spot, frogeye leaf spot, and blue mold. Wider spacing allows for more sunlight, better air flow through the canopy, and better drying conditions for the foliage at the bottom of the plant.
Balanced Fertilization
Disease-causing organisms are generally favored by imbalanced nutrients that may cause poor or irregular growth or prematurely senescent tissues. Some pests, such as root-knot nematodes, are favored by deficiencies in nutrients such as potassium. Other causal agents, including the black shank fungus, are favored by excessive nitrogen. Usually, a healthy crop is one that has received balanced fertilization—neither excessive nor deficient.
Prevent Soil Movement
Soilborne pathogens spreads whenever infested soil is moved on machinery and other equipment, by water washing soil from one part of the field to another, by moving transplants with infested soil around the roots, or by any other means by which infested soil is moved. Regardless of whether disease is present, soil movement between fields should be avoided. After cultivation, wash equipment with a detergent solution of the same strength used to wash clothes. Cultivate diseased fields last to reduce the chance of spreading the disease organisms to “clean” areas.
Common Tobacco Diseases
Diseases in Greenhouses
The most common diseases in greenhouses are caused by Rhizoctonia (damping off), Sclerotinia (collar rot), Pythium (damping off), Berkeleyomyces (black root rot), and bacterial soft rot (Pectobacterium spp.). Rhizoctonia generally causes damping-off observed before clipping begins, and Sclerotinia causes damping-off after clipping. Damping-off caused by Pythium is preceded by extensive yellowing of the plants. TMV is rare under good sanitation practices, but it is devastating where it occurs.
Sanitation Practices
Mowers can spread tobacco mosaic virus and bacteria. Wash and sanitize blades and the underside of the deck before each clipping of each greenhouse. Furthermore, be sure the mower thoroughly removes clipping debris (usually by vacuum). Clipping too much of the plant in one pass or allowing mower bags to get too full causes more debris to fall back into the trays. Leaf debris in the trays or on the plants increases humidity in the plant leaves and is associated with collar rot and bacterial soft rot.
Before using trays that have been used before, thoroughly wash them and allow them to dry. Do not depend on dipping trays in any sanitation product, including bleach, to kill pathogens satisfactorily. Steaming trays at 176°F for 30 minutes is an excellent alternative to fumigation. Steaming trays at temperatures slightly below 176°F (but no less than 158°F) for two hours can be used to get similar sanitation control as at 176°F. Tray steaming will not destroy black root rot or TMV. Growers who know greenhouse transplants were a source of TMV or black root rot should dispose of the trays that were used to produce the infected transplants and purchase new ones.
Environmental Conditions
Greenhouses should be fully ventilated when temperatures are not cold enough to damage the plants. Furthermore, to remove humidity from the greenhouse, place fans just above the plant canopy to circulate air around the structure. Polytubes or other power ventilators can also be used to remove humidity. Ventilation will help to reduce leaf moisture and subsequent disease. Pythium is most damaging at pH levels above 6.1 and at float water temperatures above 68°F. To keep the water temperature cool for as long as possible, do not fill the bays with water until it is time to float the trays. Closing greenhouses during July or August to allow temperatures to reach 140°F for eight hours per day for seven days helps kill pathogens. Heat-sensitive items should be removed, and adequate moisture should be maintained in the house.
Other Precautions
Never dump plants or used media within 100 yards of a greenhouse. Once diseased plants have been dumped, they may serve as a source for collar rot for up to five years. Walkways and entryways should be made of gravel, asphalt, concrete, or other material that can be easily washed. Boots worn outside the structure should not be worn inside unless they have been sanitized. Use special care in preventing field soil from contaminating water beds in float systems. Also, do not use pond water in beds because it can be a source of disease inoculum. Excessive or splashy watering, poor drainage, plant injury, overcrowding, and excessive humidity most often lead to disease problems in greenhouses. Use only media produced for tobacco transplants. Do not introduce tobacco products into the greenhouse. Do not allow weeds, especially horsenettle, to grow in or around the greenhouse.
Tobacco should not be grown for any reason during a three-month period between October and February to ensure that blue mold, especially a Ridomil-resistant strain, does not overwinter. Should blue mold be a concern, spray Dithane Rainshield weekly after plants reach the size of a quarter to help prevent disease.
Black Shank
Black shank is caused by a soil-inhabiting, fungal-like organism (Phytophthora nicotianae) that belongs to a group of the most destructive plant pathogens, oomycetes. This group of pathogens thrives in high-moisture climates. The black shank pathogen produces three types of spores, including survival structures (chlamydospores) that can survive in soil from four to six years, so longer crop rotations are more effective at reducing diseases. Avoid moving soil around the state. When conditions are conducive for disease, the pathogen produces motile, swimming spores (zoospores) that infect tobacco roots and sometimes infect stalk stems at leaf scars (where leaves fall off ). Irrigation and rain can splash spores onto leaves, causing leaf infections that develop as brown, circular spots.
The symptoms of black shank are characterized by yellowing and wilting of leaves. Once infection occurs, death usually follows quickly. In highly resistant varieties, the symptoms on the stalks are usually confined to near-ground level. When stalks are split, the pith often appears blackened and separated into discrete discs. Although the presence of discs is not solely diagnostic of black shank and can occur because of other factors (such as lightning damage); not all plants suffering from this disease exhibit this symptom. Rotation and varietal resistance are usually integrated into a management program to reduce damages caused by black shank.
There are different sources of genetic host resistance used in available varieties. Polygenic resistance, resistance that comes from multiple genes, has been the predominant form of resistance for many years. It is effective to varying degrees against both race 0 and race 1 of black shank fungus. Many commercial flue-cured varieties have some level of polygenic resistance. For example, K 346 has a high level, while K 326 has a low level. A single gene (Ph) is another incorporated form of resistance that imparts complete resistance (immunity) to race 0 of the pathogen but is susceptible to race 1. Varieties with the pH gene may vary in their resistance to race 1 depending on the polygenic resistance that is in their heritage. Currently, most varieties with the pH gene have little polygenic resistance, which means they will be more susceptible to race 1 than older varieties, such as K 346, that have high levels of polygenic resistance. Most new varieties released over the past five to 10 years have the Ph gene, similar to the proportion of varieties that currently have the MI gene for races 1 and 3 of the southern root-knot nematode. Therefore, over time, the Ph gene has become a less effective tool. Whenever varieties with the Ph gene are planted crop after crop, race 1 becomes more prevalent, even if it was not initially the predominant race. An additional resistance gene, Wz, confers resistance to race 1 and race 0. Although this new gene adds another tool, recent research has demonstrated that P. nicotianae populations can overcome resistance when challenged by repeated plantings of tobacco containing this gene. Rotation of host resistance traits may help prevent resistant pathogen populations from developing.
Blue Mold
Blue mold is caused by an airborne fungal-like pathogen (Peronospora tabacina), that caused widespread losses in North Carolina in 1979 and 1980. During those years, the disease occurred in fields as well as in plant beds. The fungus also spreads when infected seedlings are shipped. Its occurrence was sporadic until 1995, when it became widespread again. It has since become less common and has only recently been found in one county in North Carolina in 2016 and 2017.
Foliar infection by blue mold is characterized by the development of round, yellow spots with gray or bluish-gray mold on the undersides of the leaves. These spots rapidly multiply in favorable environmental conditions (high humidity and cool temperatures) and coalesce to kill entire leaves. Old blue mold lesions are tan to white. When systemic, the fungus penetrates the plant, interfering with normal plant growth and resulting in stunting, distortion, and the eventual death of the plant. Either type of infection can cause severe losses under favorable environmental conditions.
Because air currents disperse this fungus, crop rotation and stalk and root destruction do not affect this disease in North Carolina. The fungus does not overwinter in North Carolina, so predicting future infestations is not possible.
Strategies to prevent disease development depend on minimizing favorable environmental conditions, including planting in well-drained soils, maintaining appropriate plant spacing, and proper nutrition to prevent excess growth and humidity in the canopy.
Brown Spot
Brown spot is caused by an airborne fungus (Alternaria spp.). It may be considered an “opportunistic” disease-causing agent because it causes damage to senescent or damaged tissues. It does not usually become a problem in varieties tolerant to this disease when good cultural practices, such as proper planting density and canopy management, are followed. However, during periods of extended rainfall late in the harvest season, it can become destructive. It may also be destructive on prematurely senescent tissues or where plants become damaged from other environmental conditions. Infested leaves may develop rot and spots when cured, even when symptoms are not observed in the field. Ensure leaves are dry when harvested and cured and the barn is properly ventilated.
Fusarium Wilt
Fusarium wilt is caused by soilborne fungi (Fusarium spp.). It can live well on decaying organic matter in the soil and forms spores (chlamydospores) that are very resistant to adverse conditions. Fusarium wilt is not as aggressive as some other diseases, such as Granville wilt or black shank, and is considered an “opportunistic” disease. If tobacco plants are stressed, such as by root wounding, other soilborne diseases, or nematode infection, significant Fusarium wilt may develop. Although crop rotation and stalk and root destruction are beneficial to some extent, these practices do not drastically reduce Fusarium wilt development because of the ability of Fusarium spp. to live on organic matter and form strong, resting spores. Prevent spread through soil movement and minimize plant stress by managing nematodes and proper fertilization.
Granville Wilt
Granville wilt, caused by a soilborne bacterium, Ralstonia solanacearum, is common in North Carolina, and symptoms appear first as a wilting on one side of the plant. As the disease progresses, the entire plant wilts and eventually dies. When plants do survive, they are usually stunted and their leaves may be twisted and distorted. The stalk usually becomes dark, especially at the ground level. At this stage, Granville wilt may be easily confused with other diseases, such as black shank.
A diagnostic characteristic of Granville wilt is the streaks that extend up the stalk just beneath the outer bark.
Infection occurs when these microscopic bacteria enter wounds or openings in the root system. While the roots may become damaged as they grow through the soil, cultivation, and nematode damage can cause further damage and increase the incidence of this disease. Producers can avoid spreading Granville wilt by preventing soil movement.
Relatively high soil temperatures and adequate-to-high moisture levels in soil favor Granville wilt bacteria; wet seasons greatly increase infection by Granville wilt bacteria. Infection may not be noticed immediately because wilting symptoms may not appear until plants are under moisture stress. It is not unusual to observe symptoms of Granville wilt several weeks after infection initially occurs.
Granville wilt bacteria also can infect tomatoes, white potatoes, peppers, eggplants, and peanuts. Good crop rotations can include fescue, small grains, or soybeans. Ragweed, common to most of North Carolina, can also be infected and should be managed. Variety selection can help mitigate losses.
Hollow Stalk (Soft Rot)
Hollow stalk or soft rot caused by Pectobacterium spp., usually first appears near topping and suckering time. The bacteria are usually present in soil and on plant surfaces and may infect any stem wound. It is often seen in the pith at the break made by topping. Soon after infection, a rapid browning of the pith develops, followed by general soft rot and collapse of the tissue. Top leaves often wilt, and the infection spreads downward; the leaves droop and hang down or fall off, leaving the stalk bare. Diseased areas may appear as black bands or stripes that may girdle the stalk. In another phase of the disease, a soft decay appears at the junction where leaf petioles are attached to the stalk.
The bacteria may also be present on workers’ hands as they top, sucker, or harvest the crop, so proper sanitation may minimize disease. Hollow stalk is not common unless there is frequent rainfall and high humidity, which favors infection and subsequent disease development. The use of some contact sucker control agents may also lead to an increase in hollow stalk, especially if leaf axil tissue is damaged.
If affected leaves are harvested when wet and carried to the barn, they can develop barn rot during curing. Ensure leaves are dried and the barn is properly ventilated to minimize barn rot.
Pythium Stem Rot
This disease is caused by a group of Pythium species that include Pythium aphanidermatum, the most important and aggressive species, followed by P. ultimun var. ultimun and P. myriotylum. Pythium was believed to affect only tobacco seedlings in the early stages of growth after being transplanted in the field, causing damping-off, root and stem rot, and feeder root necrosis. Symptoms of Pythium stem rot are very similar to those caused by black shank, making loss estimates difficult. In most cases, Pythium stem rot affects some roots at the soil line level and most of the lower stem, causing a sunken black lesion that will continue to grow upward in the stem. Wilting of plants and chlorosis are also observed in plants affected by Pythium.
The predominant Pythium species (P. aphanidermatum) has not been detected on tobacco transplants produced in greenhouses in North Carolina; thus, the potential of carrying Pythium-infected transplants with this pathogen from greenhouses is minimal. However, other Pythium species can be carried on infected transplants from the greenhouse and cause seedling blight. Spores of P. aphanidermatum can survive in the soil and plant debris in the field and can infect a large number of host plants, including peppers, tomatoes, corn, cucumbers, and peanuts, among others.
High temperatures and soil moisture favor the development of Pythium stem rot. Because the incidence of this disease depends on environmental conditions, the development of control strategies is very difficult to generalize. Management of Pythium is similar to that for black shank, although resistance to this disease has not been identified.
Root-Knot Nematodes (and Other Nematode Problems)
Nematodes are microscopic roundworms that require living plant tissue to survive and complete their life cycle. Nematodes that attack tobacco live in the soil and are spread when infested soil is moved. The key to nematode control is to keep populations at nondestructive levels. Although a single nematode is not harmful, high populations have a devastating effect. Under favorable conditions, root-knot nematodes complete their life cycle in only three weeks. Thus, in North Carolina they can produce as many as seven generations during one tobacco-growing season.
The most important nematode on tobacco in North Carolina is the southern root-knot nematode, Meloidogyne incognita. However, populations of other Meloidogyne species are increasing in this state, especially M. arenaria (peanut root-knot nematode), M. javanica (Javanese root-knot), and M. hapla (Northern root-knot nematode), which are severely damaging. The spread of M. javanica and M. hapla is a threat to root-knot control in the state because of the lack of resistance to them and the possibility that some non-fumigant nematicides may not effectively control them. Also, certain races of M. incognita that can attack root-knot-resistant varieties appear to be increasing in the state. More recently, M. enterolobii (guava root-knot nematode) has been introduced into North Carolina, and has been confirmed in 16 counties, mainly in the central coastal plains. This nematode species is particularly aggressive and difficult to control. If M. enterolobii is suspected in a field, contact local N.C. Cooperative Extension agent, and submit a sample for DNA confirmation to the NCDA&CS Agronomic Services–Nematode Assay Lab.
To determine the infestation level of root-knot nematodes, examine the roots and have soil assays completed. First, observe the roots at random just after fall stalk and root destruction (immediately after harvest). You can estimate the infestation level by observing the area galled and using the index below. Galls will appear as bumps or “knots” along a normally smooth root.
- Low infestation—0% to 10% of root area covered with galls
- Moderate infestation—11% to 25% of root area covered with galls
- High infestation—26% to 50% of root area covered with galls
- Very high infestation—51% to 100% of root area covered with galls
The risk posed by moderate-to-high infestations is often equal to or greater than the risk posed by very high infestations. Even low-to-moderate infestations on a nematode-resistant variety warrant rotation to a non-host crop. The higher the gall index, the higher the infestation level. You can learn much about the root-knot population in each field by systematically assessing such indices from several locations in each field. This information will prove valuable when making decisions about the use of root-knot-resistant varieties.
To obtain nematode assays for all nematode species, take soil samples from the field and send them to the Agronomic Services, Nematode Assay Section, North Carolina Department of Agriculture and Consumer Services, 4300 Reedy Creek Road, Raleigh, NC 27607-6465. The general procedure for collecting a soil sample for a nematode assay is outlined by the NCDA&CS. These samples must be taken in the fall (before December 1) to provide reliable information and guide nematode management decisions for the following cropping cycle. No more than five acres should be represented by one sample, which should consist of at least 20 cores or subsamples taken from 6 to 8 inches depth. Gently and thoroughly mix the collected soil cores in a clean bucket, and place a 500cc portion (approximately 1 pint) into a plastic bag, and then into a Nematode Assay Lab submission box. Label the box with an identifying code that will help you remember where the sample was collected. Samples must not be allowed to dry or heat above 80°F. Do not freeze the soil samples. The counts obtained from samples taken in the spring are usually much lower and are therefore not nearly as reliable. Numbers reported to you and your local Extension agent represent the number of nematodes per pint of soil.
As with other tobacco diseases, control of root-knot and other nematodes must include a combination of suitable practices (including avoidance of soil movement), use of resistant varieties, and crop rotation. Tobacco varieties are available with resistance (conferenced by a gene called Rk) to races 1 and 3 of M. incognita and race 1 of M. arenaria, which are listed in NC State Extension’s Flue-Cured Tobacco Guide (AG-187). Currently, nearly all the commercially available tobacco cultivars sold for growing in North Carolina and South Carolina possess this resistance. However, no commercial tobacco varieties with resistance to M. enterolobii are available at this time. Non-host and poor-host crops for root-knot nematodes, such as southern and guava root-knot nematodes include sorghum, grasses, grain, alfalfa, peanuts, and resistant varieties of soybean and corn (Table 6-10).
| Root-knot nematode species | Does this root-knot species infect tobacco? | Other host crops | Non-host or poor-host crops |
|---|---|---|---|
| Southern root-knot nematode (Meloidogyne incognita) | Yes [1] | Soybean, cotton, corn, small grains (wheat, oats, rye), tomato, watermelon, sweetpotato, potato, other vegetables | Peanut, sesame, sunn hemp |
| Peanut root-knot nematode (Meloidogyne arenaria) | Yes [1] | Peanut, soybean, corn, small grains, sweetpotato, tomato, watermelon, sweetpotato | Cotton, strawberry, sesame, sorghum-sudangrass |
| Javanese root-knot nematode (Meloidogyne javanica) | Yes | Soybean, cotton, corn, small grains, sorghum, tomato, watermelon, potatoes | Peanut, pepper, sunn hemp |
| Northern root-knot nematode (Meloidogyne hapla) | Yes | Soybean, peanut, sweetpotato, tomato, potato, other vegetables | Cotton, corn, watermelon, small grains, sesame, sorghum-sudangrass |
| Guava root-knot nematode (Meloidogyne enterolobii) | Yes | Soybean, cotton, sweetpotato, tomato, potato, other vegetables | Corn, peanut, small grains, sesame, sorghum-sudangrass |
[1] Genetic host resistance is available in some tobacco varieties to this species. ↲
Target Spot
Target spot (Rhizoctonia spp.) has been prevalent in North Carolina since 1984, especially in plant beds and greenhouses. In 1995, it caused the greatest losses of any disease since 1959. The soilborne fungus persists in the soil through survival structures (sclerotia), which produce hyphae or spores (basidiospores) in warm, humid-to-wet environments. The spores will spread through the air or rain splashing. Saturated soils and leaf moisture favor sporulation of the fungus and germination of the spores into the tobacco leaves.
Target spot symptoms resemble those of brown spot. With target spot, the centers of the lesions rapidly become very thin and shatter if only slight pressure is applied. Both brown spot and target spot can form concentric rings. Because target spot lesions are so fragile, the necrotic areas usually drop from the leaf, leaving a ragged appearance. Target spot may occur on leaves at any plant position and, where conditions favor the problem, may cause considerable destruction. Target spot, like brown spot, is favored by frequent rainfall and high humidity. Removing the lower leaves and ensuring adequate nitrogen are recommended management tactics.
Tobacco Mosaic Virus
Tobacco mosaic virus (TMV) is one of the most contagious tobacco diseases that growers encounter in North Carolina. The virus that causes it is large, and like all other viruses requires living tissue to multiply. Once a TMV particle enters the plant, it becomes a part of that plant and will persist in the plant tissue. TMV is spread in the sap of diseased plants. Anything that moves sap or fluids from a diseased plant to a healthy plant will move the virus. That includes machinery used during cultivation and the hands and clothing of workers. It is not spread through air currents or by other carriers associated with most other diseases.
TMV is not as sensitive to weather conditions as most other tobacco diseases; however, it is easier for plants to become infected when there is moisture on them, and they are succulent and growing rapidly. Damage is most severe when plants are infected during hot, dry conditions.
The most common symptom of TMV is leaf mottling, which is alternating areas of light and dark green tissue. This symptom is especially noted in the top of the plant or in younger tissue. During periods of high temperatures and high light intensity, affected portions of leaves may die, resulting in “mosaic burn.”
Because of the virus’s unique nature, control of TMV must be approached differently from that of other diseases. No chemicals are labeled for mosaic control, although the milk-dip treatment is beneficial as workers perform tasks within the crop. Resistant varieties are very valuable control tools (see Table 6-9).
Also, field rotations, clean equipment, and discarding of seedling trays (if TMV incidence was at least 20% by layby in any field) is important to manage TMV. In addition, greenhouse clippers, transplanters, tractor bottoms and tool bars, and any other equipment that came in direct contact with the foliage should be washed and sanitized with a bleach solution of 25% to 50%.
Tomato Spotted Wilt Virus
Tomato spotted wilt virus (TSWV) is a potentially devastating disease of tobacco in North Carolina. This virus is also destructive in North Carolina tomatoes, peppers, peanuts, and white potatoes. The host range is large, including many weeds and ornamentals. TSWV is moved from plant to plant by thrips. In most years, the tobacco thrips is the most important vector of TSWV early in the field season; however, the western flower thrips can also vector this virus. Mild winter conditions allow for thrips to increase in population and increase inoculum in winter weeds, leading to increased TSWV incidence in tobacco crops. An early, dry spring causes winter hosts to yellow and die earlier than usual, causing thrips to begin moving off these dying weeds at the time that tobacco is being transplanted. Generally, tobacco seems to be most susceptible to infection at transplanting. As the crop ages, it is progressively less likely to be infected by a virus-carrying thrips. TSWV is established across North Carolina, and mitigating losses requires an integrated approach that reduces weed hosts and thrips pressure.
Symptoms of TSWV vary with plant age, virus strain, and environmental conditions. Newly transplanted seedlings die rapidly, then swiftly decay. As such, seedling infections are often misdiagnosed as other seedling diseases or transplanting problems. Plants that are ankle-high and taller will show some characteristic foliar symptoms. On small plants, dark reddish-brown specks and leaf distortion are common on the youngest leaves. Slightly older plants will show classic reddish-brown necrotic spots or ringspots, often with star-like projections into the green leaf tissue. Necrosis of tissue running adjacent to leaf veins is common and characteristic.
Despite the term wilt in the name, older plants only appear wilted because of the twisting and distortion the virus causes. Symptoms are usually most severe on one side of the plant and in the bud. Infected plants near flowering may have black streaks running down one side of the stem, often resembling burn from contact sucker chemicals. Streaks also occur within the pith. Plants that get infected near, during, or after flowering suffer little loss. Symptoms on these plants are generally local, being restricted to the leaf or leaves that were initially infected.
Although TSWV symptoms are somewhat characteristic, the disease can be confused with other viruses, especially tobacco streak virus (TSV). TSWV is usually randomly distributed throughout a field, whereas TSV is usually very concentrated near a particular field border. The only way to be sure which virus or viruses are present is to use a reliable assay procedure to identify the virus.
Several plant species can be infected by TSWV; however, some are much better hosts than others. Research indicates that the most important sources for infection of tobacco are several species of winter weeds. Some of these include the annual smallflower buttercup, mouse ear chickweed, common chickweed, and spiny sowthistle, as well as the perennials dandelion and Rugel’s plantain. As the winter annuals begin to die in the spring, adult thrips are forced to move to alternative plants, including tobacco. If the plant on which they developed was infected, they carry the virus with them. The virus can also move back and forth between winter annuals and summer annuals and perennials.
Avoid planting during peak thrips flights; the tobacco thrips tool can help to predict periods of high risk for TSWV transmission for a specific field. Destruction of winter weeds should not occur within four weeks of setting tobacco plants, as this can cause thrips to fly into susceptible tobacco transplants.
Weather Fleck
Weather fleck is not an infectious disease, but it causes dark, metallic-like, sunken leaf spots (flecks) that gradually fade to white with age. Symptoms are most obvious on older leaves of young plants or on middle-aged leaves of older plants. Spots are often more common near leaf tips, and damage can be severe enough to blight bottom leaves. Weather fleck is an injury caused by the common air pollutant ozone. Ozone is heavy oxygen (O3) and is produced by internal combustion engines and by certain manufacturing processes. During periods of cloudy, overcast, or rainy weather, the concentrations of ozone that would normally escape into the stratosphere are held closer to ground level. During these conditions, leaf pores (stomata) remain open the longest and the leaves absorb the most ozone. Some varieties are much less sensitive to weather fleck than others, and growers who experience chronic difficulty should select a variety that is more tolerant.
A Precautionary Statement on Pesticides
Pesticides must be used carefully to protect against human injury and harm to the environment. When possible, use different modes of action when repeated application of pesticides is necessary for controlling disease. Accurately diagnose pest problems and select the proper pesticide if one is needed. Follow label-use directions, and obey all federal, state, and local pesticide laws and regulations.
| Product Name | Active Ingredient(s) | Rate(s) [2] | Company | Target [3] |
|---|---|---|---|---|
| AzaGuard | Azadirachtin 3.0% | 2–4 fl oz/10,000 sq ft. | BioSave Systems | Nematodes |
| Aleo | Garlic Oil 78.0% | 3–12 fl oz/a | Brandt Organics | Foliar diseases |
| Camelot 0 | Copper Octanoate 10.0% | 50.8 gal/a | SePro | Foliar diseases |
| Cueva | Cooper Octanoate 10.0% | 50.8 gal/a | Certis | Foliar diseases |
| DoubleNickel LC | Bacillus amyloliquefaciens strain D747 98.85% | 0.5–6 qt/a | Certis | Foliar diseases |
| DoubleNickel55 | Bacillus amyloliquefaciens strain D747 25.0% | 0.25–3 lb/a | Certis | Foliar diseases |
| Howler | Pseudomonas chlororaphis strain AFS009 | 2.5–7.5 lb/a | Agbiome | Soilborne and Foliar diseases |
| Jet-Ag | Hydrogen Peroxide 26.5% and Peroxyacetic Acid 4.9% | 3.9–7.8 fl oz/5 gal | Jet Harvest Solutions | Foliar diseases |
| Kaligreen | Potassium bicarbonate 81.9% | 2.25–3 lb/a | OAT Agrio Co. | Foliar diseases |
| LifeGard WG | Bacillus mycoides isolate J 40% | 4.5 oz/100 gal | Certis | Foliar diseases |
| Majestene | Burkholderia spp. strain A396 94.46% | 4–8 qt/a | Marrone Bio Innovations | Nematodes |
| MeloCon WG | Paecilomyces lilacinus strain 251 6.0% | 2–4 lb/a | Certis | Nematodes |
| MilStop | Potassium bicarbonate 85.0% | 2–5 lb/a | BioWorks | Foliar diseases |
| Molt-X | Azadirachtin 3.0% | 8 fl oz/100 gal | BioWorks | Nematodes |
| Nordox 75 WG | Cuprous Oxide (Cu2O) 83.9% | 1.25–4.0 lb/a | Nordox | Foliar diseases |
| OxiDate 2.0 | Hydrogen Dioxide 27.1% Peroxyacetic Acid and 2.0% | 0.25–1 gal/a | BioSave Systems | Foliar diseases |
| PerCarb | Sodium Carbonate Peroxyhydrate 85.0% | 1–3 lb/100 gal | BioSave Systems | Foliar diseases |
| Purpose Plus | Hydrogen Peroxide/Hydrogen Dioxide 33.0% | 1 fl oz/gal | A Growing Alternative, Inc. | Foliar diseases |
| PVent | Gliocladium catenulatum Strain J1446 93.0% | 0.45–0.90 lb/a | BioSave Systems | Soilborne diseases |
| Rango | Cold Pressed Neem Oil 70.0% | 6 qt/a | Terramera | Foliar diseases |
| Serifel | Bacillus amyloliquefaciens strain MBI 600 | 4–16 oz/A | BASF | Foliar diseases |
| SilMatrix | Potassium silicate 29.0% | 1–4 qt/100 gal | PQ Corporation | Foliar diseases |
| Sporan EC | Rosemary Oil 16.0%, Clove Oil 10.0%, Thyme Oil 10.0% and Peppermint Oil 2.0% | 1–3 pt/a | KeyPlex | Foliar diseases |
| Stargus | Bacillus amyloliquefaciens strain F727 96.4% | 2–4 qt/a | Marrone Bio Innovations | Foliar diseases |
| TerraNeem EC | Cold Pressed Neem Oil 84.9% | 5 qt/a | Terramera | Foliar diseases |
| Theia | Bacillus subtilis strain AFS032321 | 1.5–5 lb/a | Agbiome | Soilborne and Foliar diseases |
| Trilogy | Clarified Hydrophobic Extract of Neem Oil 70% | 0.5–2.5 gal/a | Certis | Foliar diseases |
| ZeroTol 2.0 | Hydrogen Peroxide 27.1% and Peroxyacetic Acid 2.0% | 32–64 fl oz/ 100 gal | BioSafe Systems | Foliar diseases |
| Zonix | Rhamnolipid Biosurfactant 8.5% | 45–76 fl oz/a | Jeneil Biosurfactant Company | Foliar diseases |
[1] Products named in this guide are included for informational use only. Their inclusion does not serve as an endorsement for use or efficacy. These products may not have been evaluated by university or third-party trials. The label provides all requirements and recommendations for use; deviation from the label is unlawful. ↲
[2] Rates are taken from the label at the time of publication and are subject to change. Consult the label for the most accurate rates for a given treatment. ↲
[3] Target pests may not be represented on the label. Consult the label for additional information about pests controlled with a given pesticide. ↲
Acknowledgment of Previous Contributing Authors
Hannah Burrack, Former Entomology Extension Specialist, NC State University
Lindsey Thiessen, Former Plant Pathology Extension Specialist, NC State University
Publication date: March 19, 2024
AG-660
Other Publications in North Carolina Organic Commodities Production Guide
- Chapter 1: Introduction
- Chapter 2: Organic Crop Production Systems
- Chapter 3: Crop Production Management - Corn
- Chapter 4: Crop Production Management - Wheat and Small Grains
- Chapter 5: Crop Production Management - Organic Soybeans
- Chapter 6: Crop Production Management - Flue-Cured Tobacco
- Chapter 7: Crop Production Management - Peanuts
- Chapter 8: Crop Production Management - Sweetpotatoes
- Chapter 9: Soil Management
- Chapter 10: Weed Management
- Chapter 11: Rolled Cover Crop Mulches for Organic Corn and Soybean Production
- Chapter 12: Organic Certification
- Chapter 13: Marketing Organic Grain Crops and Budgets
- Chapter 14: Organic Market Outlook and Budgets
- Chapter 15: Resources for More Information on Organic Commodity Production
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.