The sweetpotato flea beetle is the most common pest of sweetpotato in North Carolina. Soil-inhabiting pests lower grades or make potatoes unmarketable and are most economically important. Nationwide, the sweetpotato weevil is the most damaging pest of this crop. However, it is only a potential threat here, as the North Carolina Department of Agriculture & Consumer Services Sweetpotato Weevil Program has a quarantine regime that has successfully combatted this pest. Many pests attack sweetpotato foliage. In the plant bed, this injury can be threatening. In the field, however, there is little evidence that foliar pests do enough damage to warrant treatment. When dry weather coincides with low populations of parasites and predators, sweetpotato whiteflies may become abundant enough to stunt plants and directly affect harvest weight. They are perhaps even more damaging as potential vectors of several sweetpotato diseases.
A. Pests that feed on aboveground plant parts
- Caterpillars with three pairs of legs and five pairs of prolegs
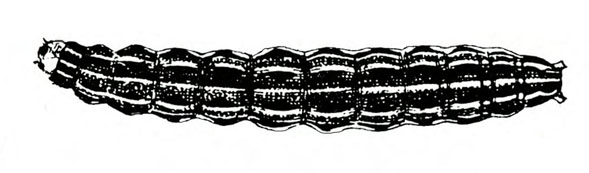
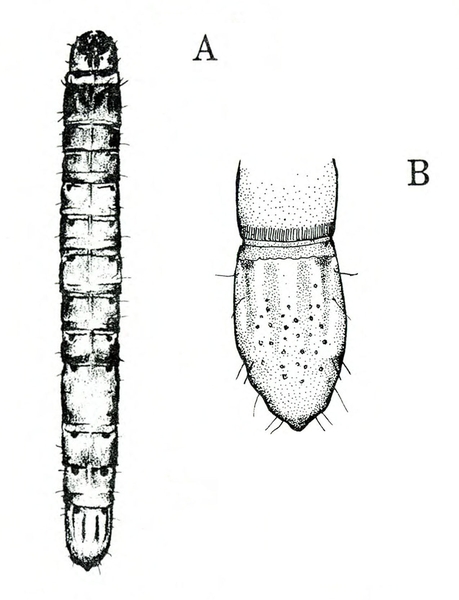
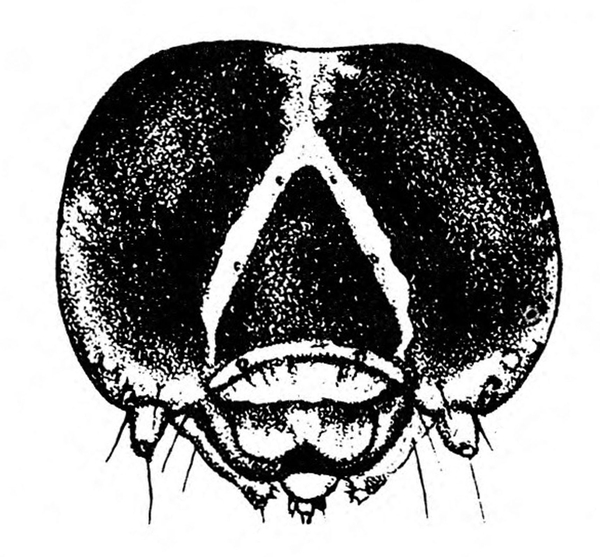
- Southern armyworm—The gray or nearly black larva grows up to 1 3/8 inches long. It has a greenish or pinkish tint, light-colored longitudinal stripes, and paired triangular spots down the back. The pale-yellow head capsule has bright-reddish-brown markings (Figure 1A and Figure 1B). This pest feeds on leaves, tender stems, and tips of branches. Larvae congregate around bases of plants during hot portions of the day.
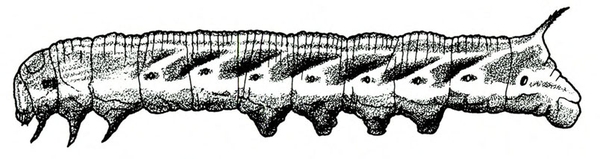
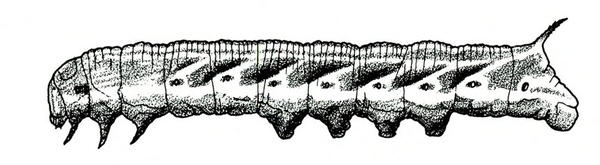
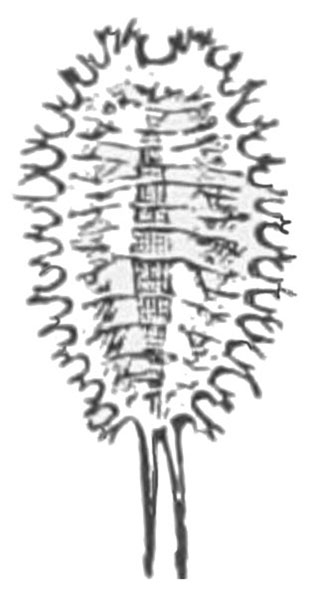
- Sweetpotato hornworm—The first instar larva is white with a black anal horn. Later instars are green or brown with black, angled marks down each side and a black anal horn. The body is up to 3 1/2 inches long. Its head is green or brown with black stripes (Figure 2). Larvae defoliate plants. They often hide near the bases of plants under large leaves.
- Yellowstriped armyworm—The pale-gray to black caterpillar is up to 1 3/4 inches long. It has yellow-orange stripes along each side and paired triangular spots on the back of most segments (Figure 3A). The head capsule is brown with black markings and a white, inverted V (Figure 3B). It feeds much like the southern armyworm.
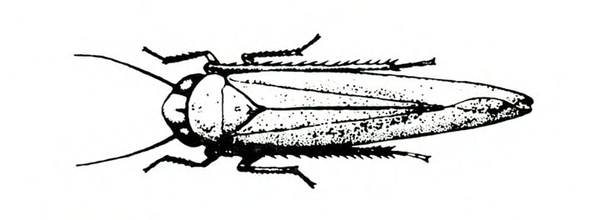
- Potato leafhopper—Up to 1/8 inch long, this spindle-shaped pest has a green body with yellowish to dark-green spots (Figure 4). It usually jumps instead of flies. It extracts sap from undersides of leaves, causing them to crinkle and curl upward. It also causes yellowing of leaf tips and margins known as “hopperburn.” Several leafhopper species attack sweetpotato. (For more information about potato leafhoppers, see “Pests of Potato.”)
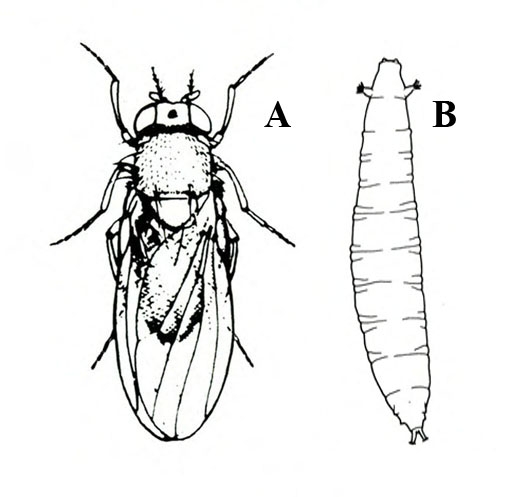
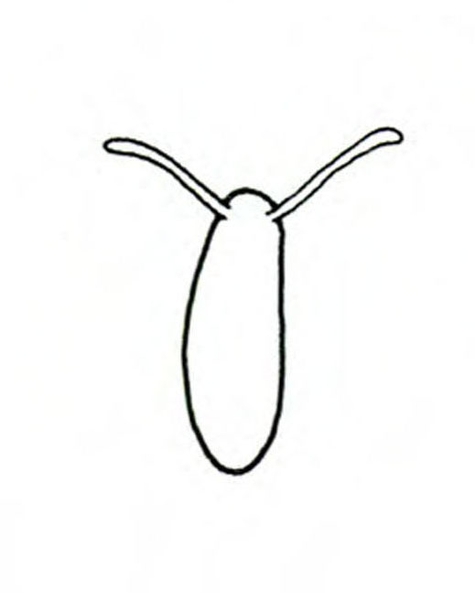
- Lesser fruit fly (or vinegar fly)—This small, yellowish fly is about 1/8 inch long and has red eyes. Flies hover around overripe or decaying produce, primarily in storage; they are often found in conjunction with their small, creamy maggots (Figure 5A–B) feeding in cracks of sweetpotatoes.
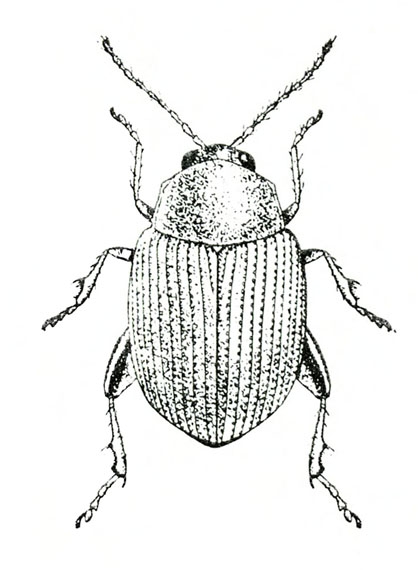
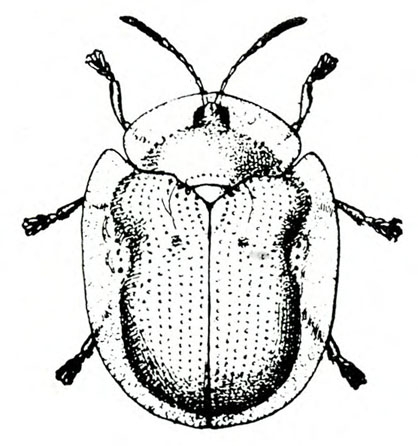
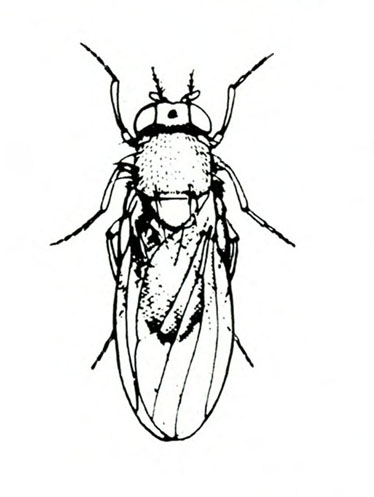
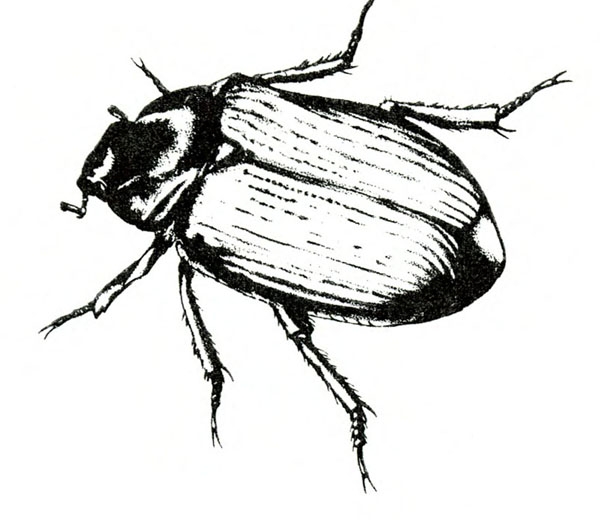
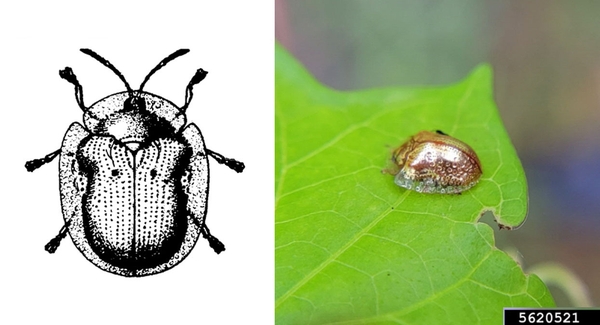
- Sweetpotato flea beetle—This black, oval beetle is about 1/16 inch long with a bronze tinge, reddish-yellow legs, and deeply ridged wing covers (Figure 6). It makes narrow channels or grooves in the upper surfaces of leaves. Injured leaves turn brown and die.
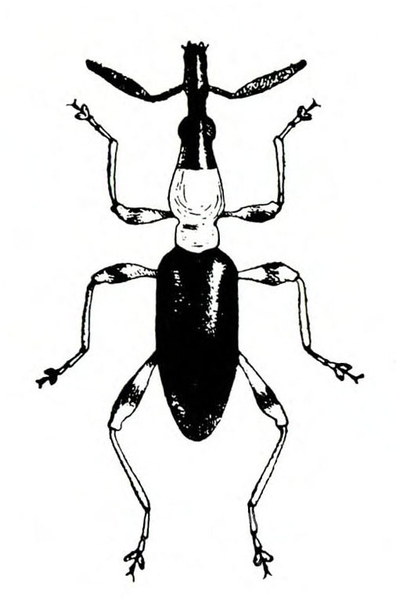
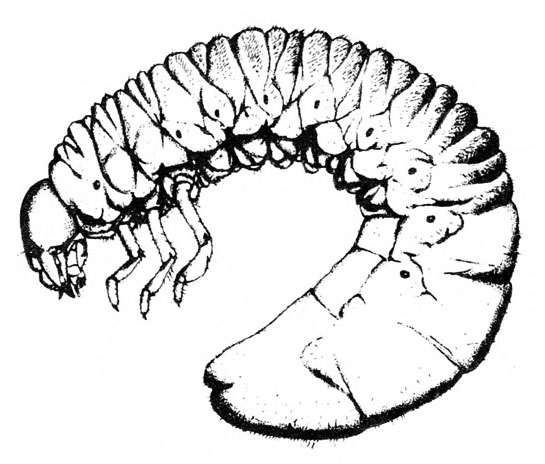
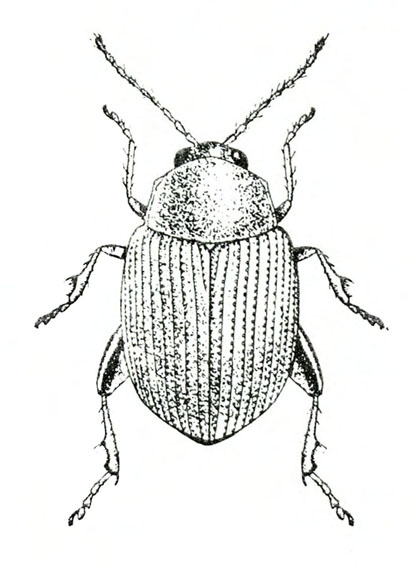
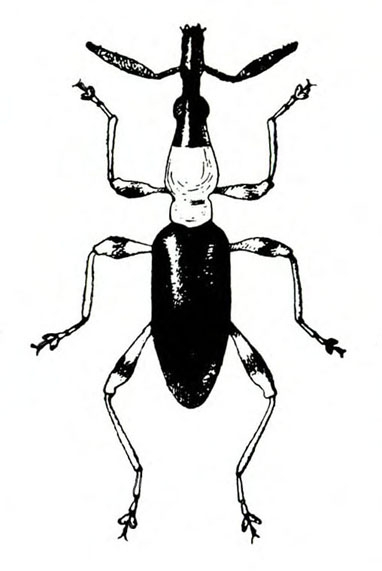
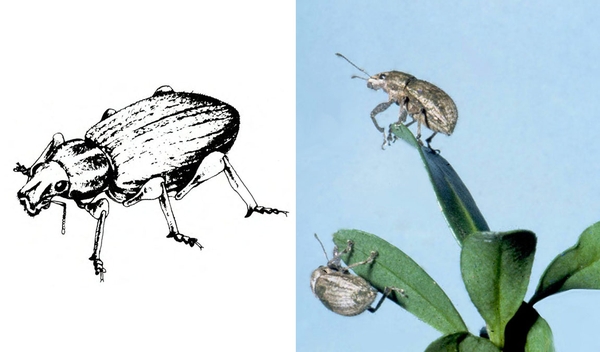
- Sweetpotato weevil—These ant-like snout beetles are about 1/4 inch long with dark-blue wing covers and red-orange legs and thorax (Figure 7). They eat foliage but primarily feed on underground plant parts. They readily drop to the soil surface when disturbed.
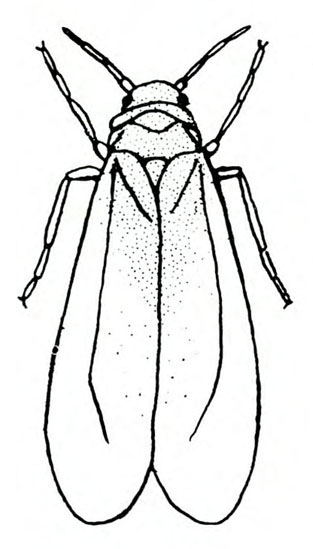
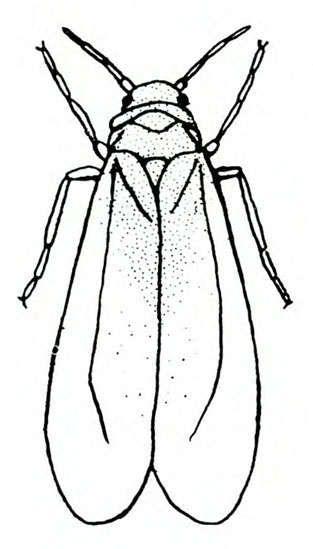
- Sweetpotato whitefly—Just less than 1/16 inch long, white, and relatively slender (Figure 8A), these are found on the undersides of leaves along with their eggs and nymphs. Young nymphs are translucent to opaque pale-yellow, flattened and scale-like, with the edges relatively near the leaf surface. Older nymphs are slightly convex and about 1/32 inch long (Figure 8B). Heavily infested plants become sticky with honeydew, pale, and stunted, and may be infected with various viruses.
- Tortoise beetles and larvae—Various species of oblong-oval, basically gold-colored beetles have varied black or red markings on their flattened, shell-like bodies, which are up to 5/16 inch long (Figure 9A). The larvae have dull-yellow, brown, or green bodies up to 1/2 inch long and black heads, legs, spots, and spines. Long spines on the larva’s abdomen hold excrement (Figure 9B). Adults and larvae chew leaves, leaving them riddled with holes.
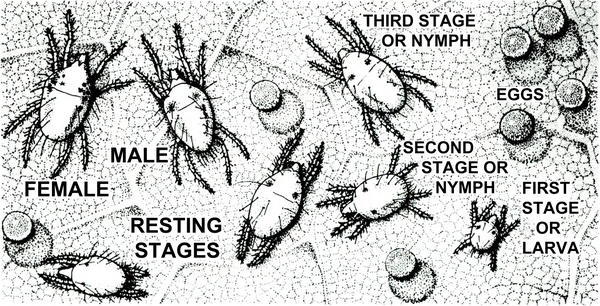
- Twospotted spider mite—Tiny (almost microscopic) and pale to dark green, twospotted spider mites have two or four dark spots. Adults and nymphs have eight legs (Figure 10). Larvae have six legs. Females are oval and about 1/64 inch long. Males taper toward the rear. Infested foliage becomes silvery because of pale-yellow stipples. Leaves eventually become pale and die. Mites spin silken webs on the undersides of leaves as they feed. (For more information about twospotted spider mites, see “Pests of Beans and Peas.”)
B. Pests that feed on belowground plant parts
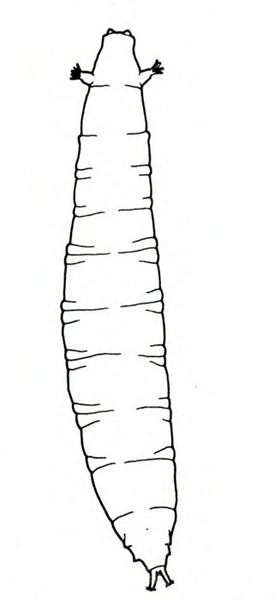
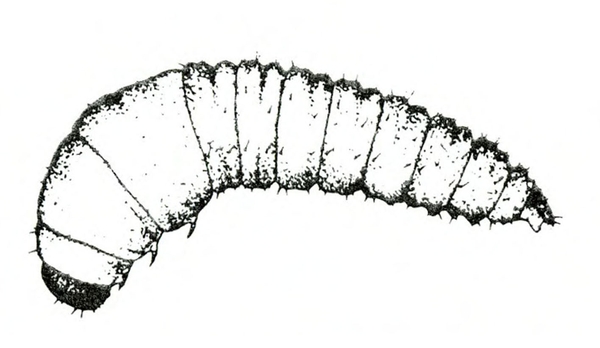
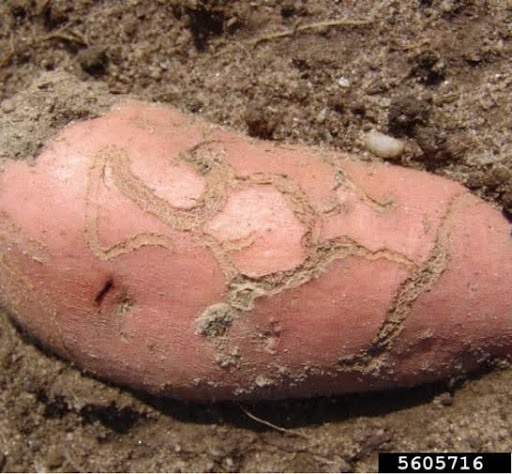
- Sweetpotato flea beetle larva—The slender, white, cylindrical larvae are up to 3/16 inch long, with three pairs of legs near the head (Figure 11). They etch shallow, winding tunnels on the surface of sweetpotatoes and their roots. The tunnels darken, split, and leave scars.
- Sweetpotato weevil adult and larva—Adults are ant-like snout beetles about 1/4 inch long with dark-blue wing covers and red-orange legs and thorax (Figure 7). The fat, legless, dirty-white larva is about 3/8 inch long with a pale-brown head (Figure 12). Beetles makes small holes on the surface of sweetpotatoes, particularly at the stem end. Larvae tunnel inside, filling tunnels with frass and causing sweetpotatoes to become bitter.
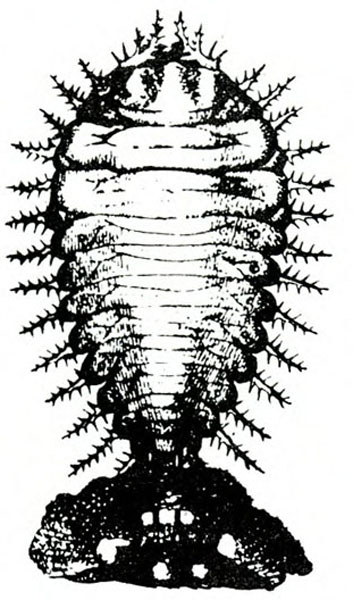
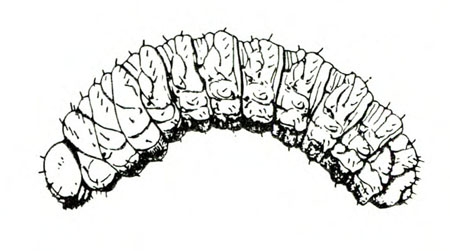
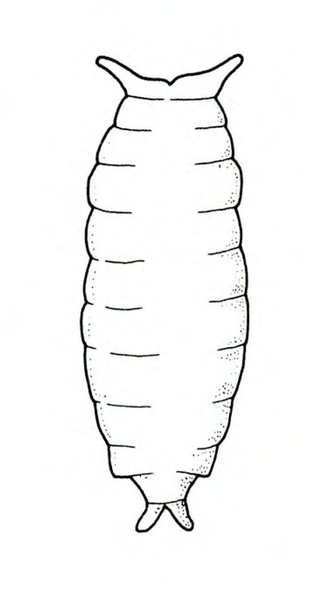
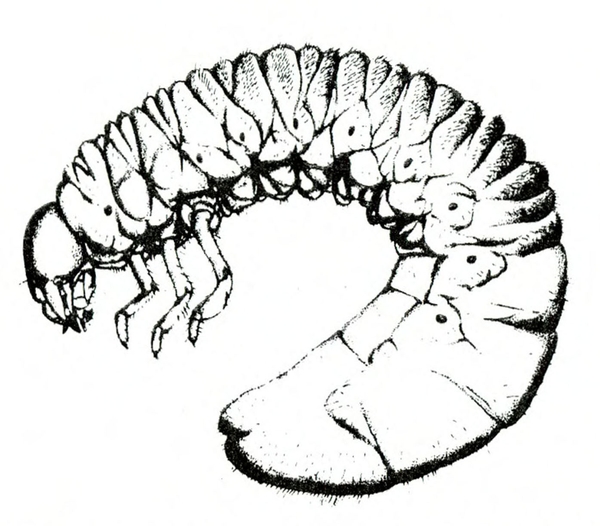
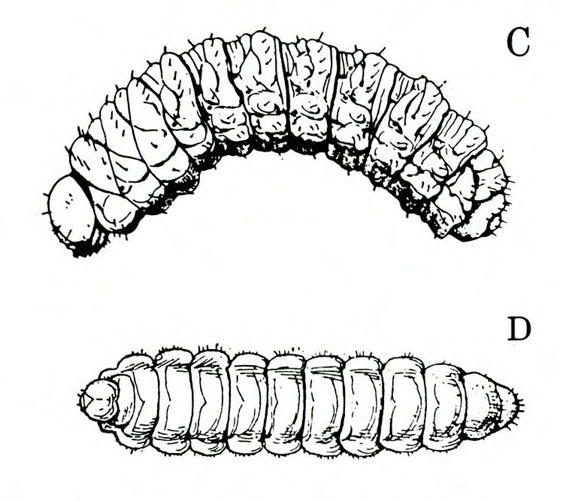
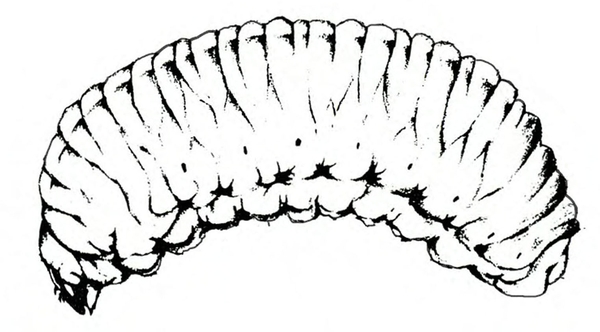
- Spring rose beetle (white grub)—The larva is a dirty-white grub up to 1 inch long with three pairs of legs near its brown head (Figure 13). It makes large, shallow feeding scars on sweetpotatoes.
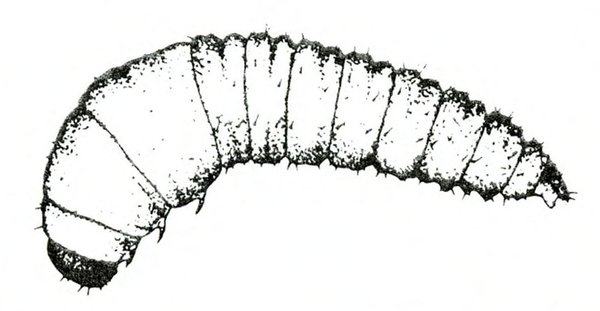
- Wireworms—These species of slender, wirelike larvae have three pairs of short legs near the head and a pair of prolegs at the tip of the abdomen. Early injury appears as large, shallow cavities in sweetpotatoes. Later damage results in deep, ragged holes (Figure 14).
- Corn wireworm—This species is yellowish brown with a dark head and a body up to 1 inch long, with scalloped edges on the last abdominal segment (Figure 15A–B). (For more information about corn wireworms, see “Pests of Potato.”)
- Southern potato wireworm—This species is cream colored or yellowish gray with a reddish-orange head, a body up to 11/16 inch long, and a closed oval notch in the last abdominal segment (Figure 15C–D). (For more information about southern potato wireworms, see “Pests of Potato.”)
- Tobacco wireworm—This species is white with a brown head, a body up to 3/4 inch long, and a V‑shaped notch in the last abdominal segment (Figure 15E–F). (For more information about tobacco wireworms, see “Pests of Potato.”)
- Whitefringed beetle larva—Up to 1/2 inch long, this yellowish-white, legless, 12-segmented grub has a small, pale head (Figure 16). Larvae gouge roots, reducing the marketability of damaged sweetpotatoes.
Lesser Fruit (or Vinegar) Fly
Drosophila melanogaster Meigan, Drosophilidae, DIPTERA
Description
Adult—About 1/8 inch long, these flies have red eyes and yellowish bodies with dark bands. Though commonly referred to as fruit flies, they are more correctly termed lesser fruit flies, vinegar flies, or pomace flies (Figure 17A).
Egg—The tiny, white, elongate eggs are almost 1/32 inch long and have two slender filaments near the head (Figure 17B). Though individual eggs are too small to be easily noticed, clusters of eggs often resemble white mold on the surface of produce.
Larva—The cream-colored maggots develop through three instars. They are about 3/16 inch long when fully grown (Figure 17C).
Puparium—Yellowish white at first, the 1/8-inch-long puparium soon turns brown (Figure 17D).
Biology
Distribution—Cosmopolitan in distribution, lesser fruit flies are most likely to congregate around piles of overripe produce or in sweetpotato storage houses.
Food Source—Lesser fruit flies and their larvae consume yeast and bacteria associated with the initial decay of plant materials. These flies are very attracted to sap flows, mushrooms, and overripe produce.
Damage—Unlike true fruit flies, lesser fruit flies do not break the skin of healthy fruits and vegetables. They breed only in cracked or decaying overripe produce. As a result, these flies and their maggots are most likely to develop large populations in cull piles, storage houses, or processing plants.
Life History—Lesser fruit flies sometimes overwinter as larvae or pupae in sheltered locations where there is an abundance of dry, fermented plant material. However, they have been known to breed throughout the winter in sweetpotato storage houses and in root cellars as far north as New Jersey. Egg-laying is significantly reduced at temperatures below 55°F or above 90°F.
Eggs are deposited in cracked produce and incubate about 24 hours before hatching. At a temperature of about 77°F, larvae feed and develop to maturity in about four days. Pupation then occurs within the shrunken skin of the last larval instar (the puparium). Adult flies emerge about five days later. Within two days, females begin ovipositing at the rate of about 25 eggs per day. This process continues for several weeks, with each female eventually producing an average of 500 eggs.
The length of a complete life cycle (adult to adult) varies with temperature. At 68°F, about fifteen days elapse, but at 85°F, a life cycle is completed in only eight days. Generations may be produced all year if the temperature is favorable and fermenting produce is available.
Control
Lesser fruit flies are subject to many natural enemies. Adults are parasitized by protozoa, fungi, nematodes, and mites and preyed upon by spiders and certain species of flies. Maggots are parasitized by certain wasps and preyed upon by staphylinid and nitidulid beetle larvae.
Infestations can be prevented by destroying piles of culled produce. Storage houses should be well screened and sealed to minimize fly entrance; still, chemical control in storage areas may be necessary. For up-to-date recommendations, consult the North Carolina Agricultural Chemicals Manual.
Southern Armyworm
Spodoptera eridania (Stoll), Noctuidae, LEPIDOPTERA
Description
Adult—The southern armyworm moth has a wingspan of 13/16 to 1 3/8 inches (Figure 18A). The forewings may vary in color from pale yellowish to dark brown. A darker streak extends from the center of each forewing almost out to the wing tip. The hind wings are white with brown veins and margins.
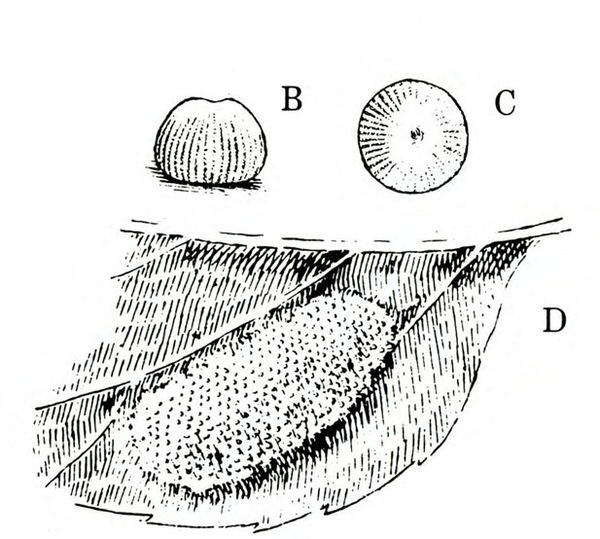
Egg—The circular, greenish egg is almost 1/32 inch wide. Viewed under magnification, the surface appears ridged (Figure 18B–D).
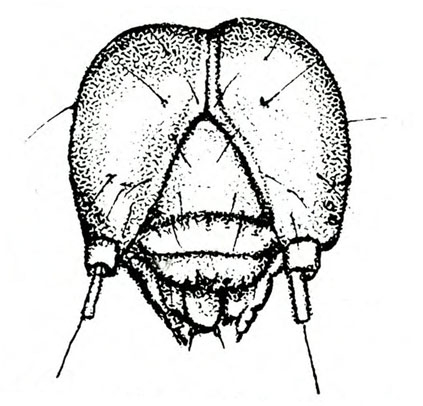
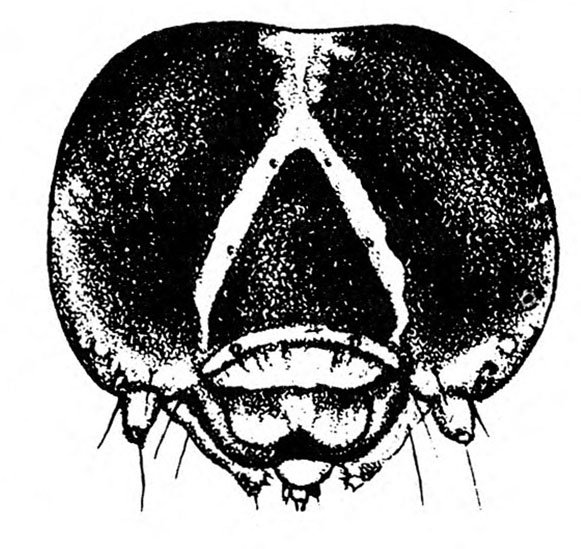
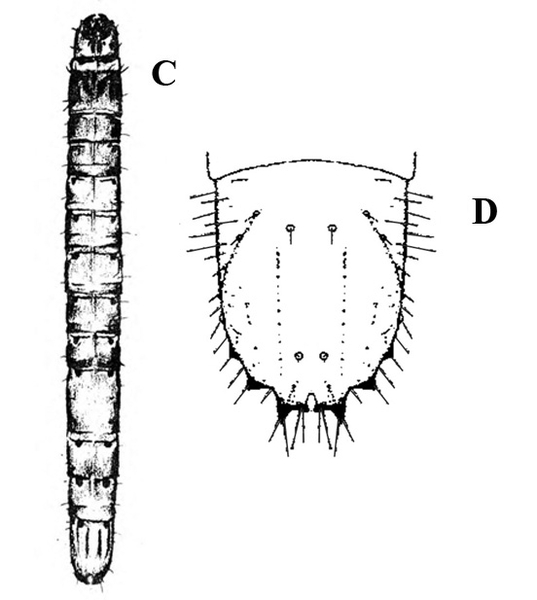
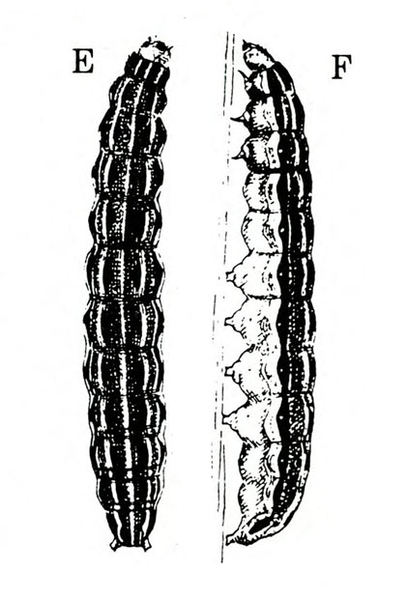
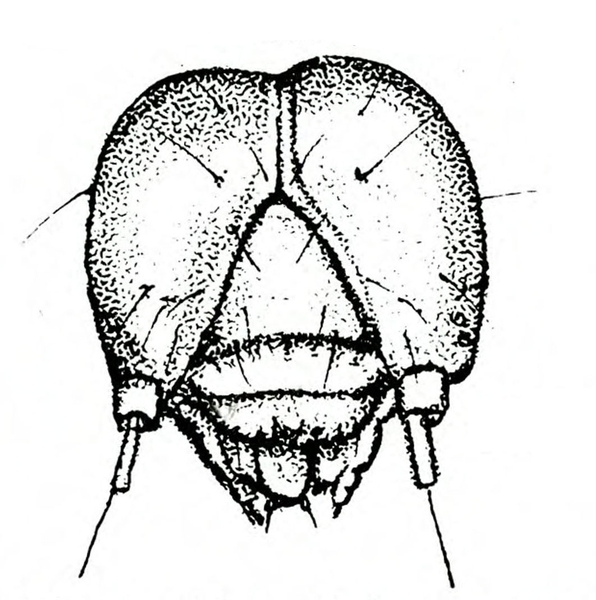
Larva—The fully grown caterpillar is gray or nearly black, with whitish stripes tinged with orange or pink, and about 1 3/8 inches long (Figure 18E–F and Figure 19). The background body color sometimes has a green or pink tint. Viewed from above, the larva has a pair of black triangular spots on each body segment except the segment near the head bearing the first pair of legs. The larva has three pairs of legs and five pairs of prolegs. The head capsule is pale yellow with bright-reddish-brown markings (Figure 18G).
Pupa—The dark-colored pupa is about 3/4 inch long and 3/16 inch wide.
Biology
Distribution—Florida, California, New Mexico, and Central America provide year-round habitat for the southern armyworm. Each year, moths migrate as far northward as Tennessee and Virginia. In North Carolina, this armyworm is only an occasional problem.
Host Plants—The southern armyworm is a general feeder with a wide host range. Weeds like spiny amaranth and pokeweed are preferred food plants. In addition to sweetpotato, vegetable crop hosts include beet, cabbage, carrot, celery, collard, corn, cowpea, eggplant, okra, pepper, potato, rhubarb, and tomato.
Damage—Though southern armyworms feed primarily on leaves, they have been known to consume tender stems and tips of branches. These caterpillars feed freely during the daytime but often are not observed because they tend to congregate around the bases of plants. Here, they gnaw on stems or feed on potato tubers or sweetpotatoes near the soil surface. During morning and evening, or on cloudy days, southern armyworms are likely to be found on foliage.
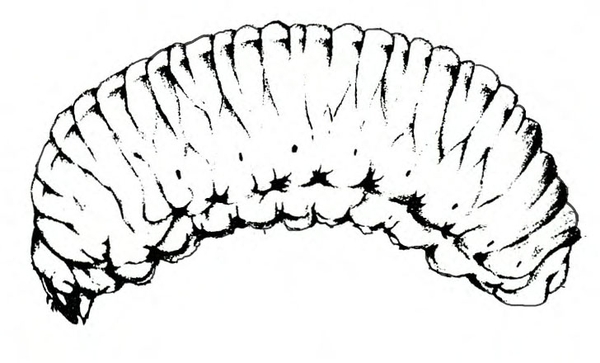
Life History—Southern armyworms overwinter either as larvae or pupae in Florida. Egg-laying moths probably arrive in North Carolina in July. Each female deposits hundreds of eggs in masses on foliage. These masses appear fuzzy because they are covered with scales from the bodies of moths (Figure 18B–D). Eggs hatch in four to six days. For about 17 days, larvae feed and develop through six instars; then they drop by means of silken threads to the soil surface, enter the soil, and pupate. Nine to thirteen days later, a new generation of moths emerges. During summer, about five weeks elapse between egg stage and adult emergence. As many as five generations occur each year in Florida, but only two or three are likely to be completed in North Carolina.
Control
The sweetpotato variety NC Porto Rico 198 has shown some resistance to the southern armyworm. Populations of this armyworm species rarely reach high enough numbers in North Carolina to warrant chemical control.
Spring Rose Beetle (White Grub)
Strigoderma arboricola Fabricius, Scarabaeidae, COLEOPTERA
Description
Adult—The slightly hairy spring rose beetle (Figure 20A) is basically greenish black with greenish-purple iridescence. The wing covers, however, are dull brownish-yellow (Figure 20B). The beetle is 3/8 to 1/2 inch long.
Egg—Oval at first, each white egg gradually enlarges, becoming more globe-shaped. From an initial size of a little more than 1/16 inch, the egg often increases to almost 1/8 inch.
Larva—The dirty-white grub has a brown head and three pairs of legs. It is almost 3/16 inch long when newly emerged, reaching a maximum length of about 1 inch and resembling most common white grubs in shape (Figure 20C).
Pupa—The pupa is white when first formed but gradually darkens as it matures. It is about the same size and shape as the adult beetle.
Biology
Distribution—Native to North America, this beetle occurs from Canada southward through Kansas and North Carolina.
Host Plants—Adult beetles have been collected from many flowers, including those of clover, rose, blackberry, timothy, wild parsnip, dogfennel, and plantain. Larvae have been observed infesting roots of sweetpotato, peanut, strawberry, and certain pasture grasses.
Damage—Grubs feed on most underground plant parts. In certain cases, they have been known to strip the taproot bare of rootlets. Sweetpotatoes injured by these grubs have large, but shallow, feeding scars.
Life History—These insects overwinter as larvae in soil. In spring, grubs hollow out elongated, slightly curved earthen cells about 15/16 inch long. Within these cells, they spend about six days as inactive prepupae and thirteen days as pupae. In Virginia, adult beetles usually emerge between May 15 and June 10. Farther north, they often do not appear before the end of June. Several days after mating, females deposit eggs singly in soil (four to five eggs per female in lab studies). Eggs hatch an average of 17 days after deposition. By the time larvae begin feeding, at least one month has elapsed since adult emergence. Only one generation is completed each year.
Control
White grub infestations are typically associated with fields formerly planted in sod or pasture. Such a relationship has not been documented for spring rose beetle grubs in sweetpotato fields, but it may exist. Chemically, spring rose beetle grubs are controlled by granular insecticides incorporated into the soil before planting. For recommended insecticides and rates, consult the North Carolina Agricultural Chemicals Manual.
Sweetpotato Flea Beetle
Chaetocnema confinis Crotch, Chrysomelidae, COLEOPTERA
Description
Adult—The tiny, oval beetle is black with a bronze tinge and about 1/16 inch long. It has reddish-yellow legs and deeply ridged wing covers (Figure 21A).
Egg—Each white, oblong-oval egg is extremely small.
Larva—The slender, white, cylindrical larva has three pairs of legs near its head. It is about 3/16 inch long when fully grown (Figure 21B). This larva has no dark spot or fleshy tubercle on its tail end like the larvae of the cucumber beetle or palestriped flea beetle.
Pupa—The pupa is white at first but gradually darkens and is about the same size and shape as the adult.
Biology
Distribution—The sweetpotato flea beetle occurs in nearly all areas of the United States where sweetpotatoes are grown.
Host Plants—The main food plants of this pest are sweetpotato, corn, small grains, bindweed, raspberry, and sugar beet.
Damage—Adult flea beetles feed on foliage, leaving narrow channels or grooves in the upper surfaces of leaves. These injured areas turn brown and die. Larvae live underground and feed on roots. Shallow, winding tunnels etched into root surfaces indicate an infestation of flea beetle larvae (Figure 21C). These tunnels eventually darken and split open, leaving shallow scars; this type of damage usually is restricted to fibrous roots. During heavy infestations, however, larvae may injure the fleshy, marketable portion of roots in the same manner.
Life History—Sweetpotato flea beetles overwinter as adults under logs and leaves, along fencerows, and at the edges of wooded areas. They resume activity in spring and begin to deposit eggs in soil near host plants. A few days later, eggs hatch. Newly emerged grubs feed for about three weeks before pupating in the soil. During summer, the entire life cycle is often completed in 30 days. Several generations per year are possible. From June onward, however, most eggs are deposited near bindweed, and flea beetle populations on sweetpotato decline.
Control
Cultural practices are instrumental in preventing flea beetle infestations. Controlling weeds along fencerows and plowing crop debris under destroy overwintering and egg-laying sites. However, planting resistant varieties such as Jewel or Centennial is the most effective means of preventing injury from sweetpotato flea beetles.
In fields with a history of flea beetle infestation, chemical control may be justified. Pre-plant, soil-applied insecticides are available. For recommended chemicals and rates, consult the North Carolina Agricultural Chemicals Manual.
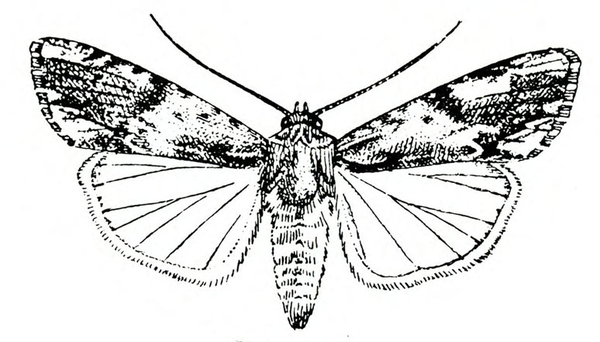
Sweetpotato Hornworm (Pinkspotted Hawk Moth)
Agrius cingulata (Fabricius), Sphingidae, LEPIDOPTERA
Description
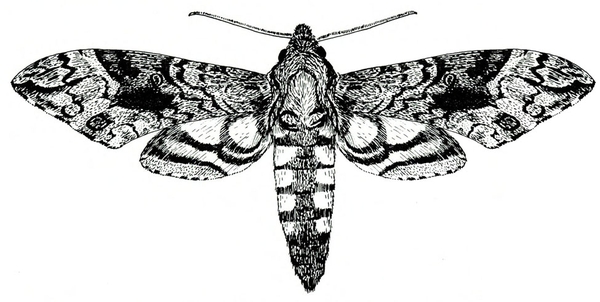
Adult—This grayish, heavy-bodied moth has a wingspan up to 4 5/16 inches (Figure 22A). The abdomen has pink spots, and the hind wings have bright-pink bands (Figure 22B).
Egg—Nearly spherical and almost 1/16 inch in diameter, the translucent egg has a slightly greenish tint.
Larva—The first instar of the sweetpotato hornworm has a white body and a black anal horn. Later instars are basically green or brown with prominent, slanted, black markings on each side of the body and a black anal horn. The head is also green or brown with three dark stripes on each side. A fifth instar hornworm may exceed 3 1/2 inches in length (Figure 22C).
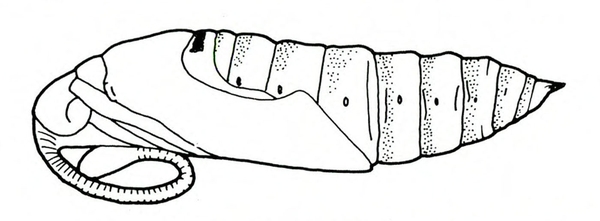
Pupa—The reddish-brown pupa is almost 5/8 inch wide and 2 1/2 inches long (Figure 22D). The large tongue case resembles a pitcher handle.
Biology
Distribution—Sweetpotato hornworms are common in tropical areas of the world and the southern United States. Moths stray as far north as Nova Scotia, but the larvae are too scarce there to become pests.
Host Plants—Sweetpotato and morning glory are the primary food plants of this hornworm, although jimsonweed has also been reported as a host.
Damage—These large worms consume much foliage, leaving only bare stems and petioles on plants. Larvae often hide under large leaves at the base of the host plant. Sweetpotato hornworms have been reported migrating en masse in Florida; however, their movement in large groups has not been observed in North Carolina.
Life History—The biology of this pest is not well documented. Its life history is probably very similar to that of tomato and tobacco hornworms. Moths appear in early June, again in August and September, and once more before frost. There are probably two and a half generations per year in North Carolina.
Control
In small gardens, you can control hornworms by picking them off plants. Chemical control, however, may be necessary in commercial production. For recommendations, consult the North Carolina Agricultural Chemicals Manual.
Sweetpotato Weevil
Cylas formicarius elegantulus (Summers), Curculionidae, COLEOPTERA
Description
Adult—This ant-like snout beetle is about 1/4 inch long (Figure 23A). The head and wing covers are metallic dark blue, and the thorax and legs are bright red-orange (Figure 23B).
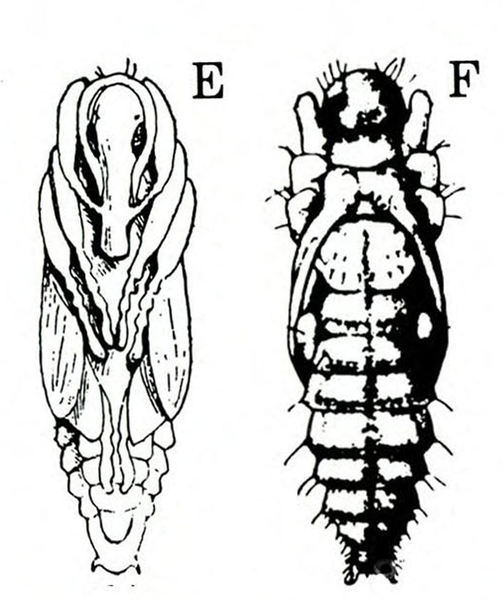
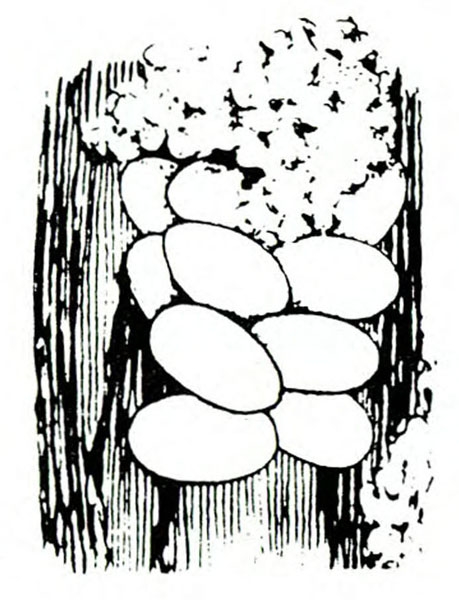
Egg—Each white or pale-yellow egg is inserted into a shallow hole in the vine. Broadly oval and almost 1/32 inch long, the egg is slightly narrower at the attached end. The dark head of the larva becomes visible inside the egg just before hatching.
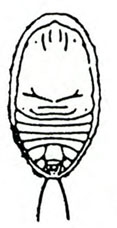
Larva—The fat, legless, slightly crescent-shaped larva has a dirty-white to gray body and a pale-brown head (Figure 23C–D). When fully grown, it is about 3/8 inch long.
Pupa—When newly formed, the pupa is the same color as the larva and about 3/16 inch long. Before it transforms into the adult, the eyes, wing pads, and legs turn dark brown, whereas the rest of the body is pale yellow. The last abdominal segment has two outward and backward curved tubercles (Figure 23E–F).
Biology
Distribution—Sweetpotato weevils are a serious problem in some coastal areas from North Carolina to Texas. Discovered in eastern North Carolina in 1967, sweetpotato weevils are now largely under control here. Rarely, sweetpotato weevils are found as far north as New Jersey.
Host Plants—Sweetpotato and related wild plants such as morning glory are the only hosts of sweetpotato weevils.
Damage—Sweetpotato weevils and their larvae are the most destructive sweetpotato pests. Infestations may reduce plant growth during the first month after planting, but other damage often is not evident until harvest. Adults feed on foliage, but they prefer to attack stems and sweetpotatoes underground. Small holes scattered over the surface of infested sweetpotatoes, particularly at the stem end, are the beetles' egg-laying or feeding punctures. Infestation causes sweetpotatoes to become bitter, rendering them unfit for human consumption or stock feed.
Life History—Beetles become active in the field as soon as host plants are available. They first feed on leaves and stems. As plant stalks enlarge and become woody, adult females prepare to deposit eggs. They make holes in stems and fleshy roots near the soil surface. Females place eggs in these holes and cover them with a jellylike secretion. Each female deposits an average of 120 eggs.
Larvae hatch less than a week after eggs are laid. They burrow deep into stems and fleshy roots—feeding for about two to three weeks. At the end of this period, third-instar larvae return to the plant surface nearest the soil line to pupate. Pupae transform into adults in about a week, but another four days often elapse before the new beetles emerge from their pupal cells. Adults live about two and a half to three months in summer and as long as eight months in winter.
Sweetpotato weevils continue to feed and breed throughout winter in stored sweetpotatoes. Development and activity, however, are much slower at temperatures below 60°F. As many as eight generations may be produced each year.
Control
Cultural practices such as crop rotation, use of weevil-free planting stock, and destruction of volunteer plants and crop residue are primary elements of sweetpotato weevil control. Planting sweetpotatoes in the same fields year after year leads to increased weevil populations. If slips for planting cannot be obtained from a weevil-free area, each sweetpotato chosen for seed should be examined carefully and destroyed if infested. Deep-rooted varieties such as Porto Rico are preferable to shallow-rooted varieties such as Gold Rush.
Insecticide treatments can be applied after harvest to prevent development of weevils in storage. Treated sweetpotatoes will need to be washed thoroughly once they are removed from storage. For recommended insecticides and rates, consult the North Carolina Agricultural Chemicals Manual.
Sweetpotato Whitefly
Bemisia tabaci (Gennadius), Aleyrodidae, HEMIPTERA
Description
Adult—The sweetpotato whitefly is slightly yellower and smaller than other whiteflies. Females are just less than 1/16 inch long, and males are slightly smaller. The head is broad at the antennae and narrow toward the mouthparts. Sweetpotato whiteflies hold their wings rooflike at about a 45-degree angle, whereas other whiteflies usually hold their wings nearly flat over the body (Figure 24A and Figure 24E). Hence, the sweetpotato whitefly appears more slender than other common whiteflies.
Egg—The 1/128-inch-long eggs are inserted on end into the undersides of new leaves (Figure 24B). The eggs are whitish to light beige, with the apex tending to be slightly darker.
Nymph—The nymphal stages are translucent to opaque pale-yellow and may have dorsal spines if the host leaf is hairy. The first nymph, or “crawler,” is no bigger than a pollen grain. The body is flattened and scalelike, with the edges relatively near the leaf surface (Figure 24C). Older nymphs are slightly convex (Figure 24D).
Pupa—The pupa (fourth nymphal instar) is beige-yellow and opaque. Pupae are plumper than nymphs, and the outer edges of the body are rounded. Some spiracular furrows have a small amount of white wax deposits. The hind setae are prominent, and the hind end is somewhat narrow. Dorsal spines are present when the host leaf is hairy and absent when the host leaf is smooth.
Biology
Distribution—Sweetpotato whiteflies occur around the world in tropical and subtropical areas and in greenhouses in temperate areas. They have been reported from all southeastern states and Arizona, California, District of Columbia, Maryland, and Texas.
Host Plants—The number of host plants is extensive. The plant families most often reported are Amaranthaceae, Compositae, Convolvulaceae, Cruciferae, Cucurbitaceae, Euphorbiaceae, Labiatae, Leguminosae, Malvaceae, Moraceae, Rosaceae, Solanaceae, and Verbenaceae.
Damage—The sweetpotato whitefly causes direct damage by removing sap and indirect damage by transporting disease. It is a vector for several important viral diseases of lettuce and melons in the southwestern United States. In addition, this whitefly can transmit sweetpotato leaf curl virus, ipomoea leaf curl virus, sweetpotato chlorotic stunt virus, and sweetpotato mild mottle virus. Both the adult and nymphal stages contribute to direct damage. Chlorotic spots sometimes appear at the feeding sites on leaves, and heavy infestations cause leaf wilting. The excretion of honeydew and the subsequent development of sooty mold fungi also may interfere with photosynthesis and other physiological functions of the plant. Even though the sweetpotato whitefly is considered a significant pest, little is known about the damage it causes or the economic thresholds.
Life History—Developmental time from egg deposition to adult emergence appears to be primarily determined by temperature, humidity, and the species of host plant. The completion of the life cycle varies from 16 to 38 days. The number of eggs laid by each female during her lifetime appears to be about 80 to 100. Crawlers hatch from the eggs and move around until they insert threadlike mouthparts into the underside of a leaf to feed. They tuck their legs and antennae underneath their bodies and settle down close to the leaf surface. Crawlers molt into scalelike nymphs that also suck sap. Nymphs molt a second and third time. The fourth stage eventually becomes a nonfeeding pupa. The adult whitefly develops within the pupa. The adult emerges through a T-shaped slit in the pupa about a month from the time the egg was laid. Females live about two weeks.
Control
Control of sweetpotato whiteflies is difficult because the eggs and older immature forms are resistant to many insecticides. In addition, the adults are extremely resistant to dry pesticide residue. For good control, the pesticide mixture must be directed toward the lower leaf surface, where all stages of the whiteflies naturally occur. Regular applications of pesticides are necessary to control crawlers and second-stage nymphs until the last of a complete generation of immature whiteflies has hatched; however, some of the pyrethroid pesticides are somewhat more effective and need not be applied as often. Neem seed extract is not as acutely toxic as some of the synthetic pesticides, but it has the advantage of being toxic to young nymphs, inhibiting growth and development of older nymphs, and reducing oviposition by adults. For specific chemical control recommendations, consult the North Carolina Agricultural Chemicals Manual.
Tortoise Beetles
Argus tortoise beetle, Chelymorpha cassidea (Fabricius), Chrysomelidae, COLEOPTERA
Blacklegged tortoise beetle, Jonthonota nigripes (Olivier), Chrysomelidae, COLEOPTERA
Golden tortoise beetle, Charidotella bicolor (Fabricius), Chrysomelidae, COLEOPTERA
Mottled tortoise beetle, Deloyala guttata (Olivier), Chrysomelidae, COLEOPTERA
Striped tortoise beetle, Agroiconota bivittata (Say), Chrysomelidae, COLEOPTERA
Description
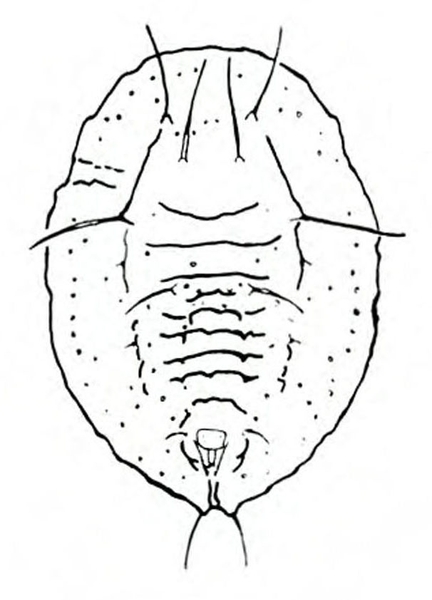
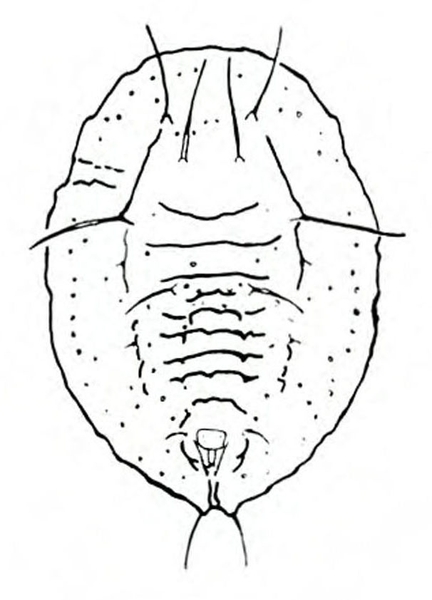
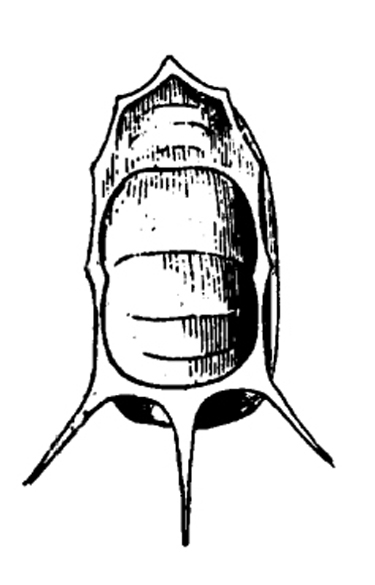
Adult—Slightly flattened and squared at the shoulders, tortoise beetles' bodies are somewhat shell-like in appearance. Body margins extend in a rooflike manner over much of the head and legs. These oval beetles are sometimes gold in color with various black or red markings, depending upon the species. Most species are 3/16 to 5/16 inch long. Argus tortoise beetles are beige with six small spots on each wing and two spots on the thorax (Figure 25A). Blacklegged tortoise beetles tend to be dark and reddish with three spots on each wing and a small, black triangle (scutellum) between the base of the wings (Figure 25E). Golden tortoise beetles are sometimes gleaming gold with transparent edges, but other specimens may be more reddish (Figure 25H). Mottled tortoise beetles are mottled by a dark blob that reaches the outer edges of the base and tip of the wings. That blob is often broken up by gold and red spots (Figure 25L). Striped tortoise beetles have five black stripes on the wings that contrast with a whitish to pale-beige background (Figure 25P).
Egg—Tortoise beetle eggs usually occur in masses. Each individual egg is attached to the plant surface by a gelatinous substance. The beige or white eggs of some species have a reddish tubercle on the upper end (Figure 25B) or various spikes (Figure 25I). Eggs are about 1/16 inch long (Figure 25M).
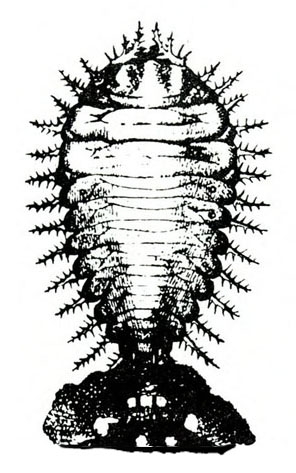
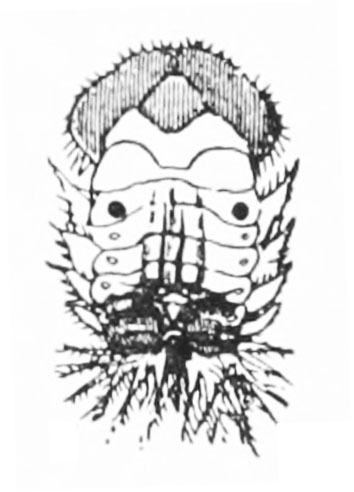
Larva—The spined larvae may be basically dull-yellow, brown, or green, depending upon the species. All have black heads, prothoracic shields (area behind the head), legs, spots, spinelike setae, and anal forks (Figure 25C, Figure 25F, Figure 25J, Figure 25N, and Figure 25Q). The anal forks are long spines near the tip of the abdomen that hold large masses of excrement. Fully grown larvae are 3/8 to 1/2 inch long.
Pupa—Pupae are oblong-oval in shape, like adult beetles, but have large, flat spines along the abdomen (Figure 25D, Figure 25G, Figure 25K, Figure 25O, and Figure 25R). Pupae are about the same size as the adults.
Biology
Distribution—Argus and mottled tortoise beetles occur in all arable sections of the United States and Canada. Golden and blacklegged tortoise beetles are most common from the Rocky Mountains eastward. The striped tortoise beetle is most common in the southern United States and Mexico.
Host Plants—Most tortoise beetles feed on sweetpotato and closely related plants like morning glory and bindweed. Argus tortoise beetles also infest cabbage, corn, raspberry, strawberry, milkweed, and plantain. Golden tortoise beetles have been found on eggplant.
Damage—Larvae and adults feed on leaves, riddling them with holes (Figure 25S). This type of damage is most harmful to seedlings or newly set plants.
Life History—Tortoise beetles overwinter as adults under bark, in leaf litter, or in other dry, protected places. In spring, beetles emerge and feed on weed hosts until sweetpotato plants are available. Female adults deposit clusters of 15 to 30 eggs on the undersides of leaves. Larvae emerge seven to ten days later. Tortoise beetle larvae all have one or two forked spikes at the tip of the abdomen on which they gather their feces. They hold the mass of feces like a parasol over their bodies and sometimes use the mass defensively against predators. After feeding for two and a half to three weeks, larvae transform into pupae. About a week later, a new generation of beetles emerges. Several generations may occur each year in southern states.
Control
Tortoise beetles and other leaf-feeding insects do not affect sweetpotato production if growing conditions are satisfactory. Cultural practices such as adequate fertilization, weed control, and well-timed planting effectively deter excessive tortoise beetle injury. Generally, chemical control is not necessary.
Whitefringed Beetles
Naupactus spp. Curculionidae, COLEOPTERA
Description
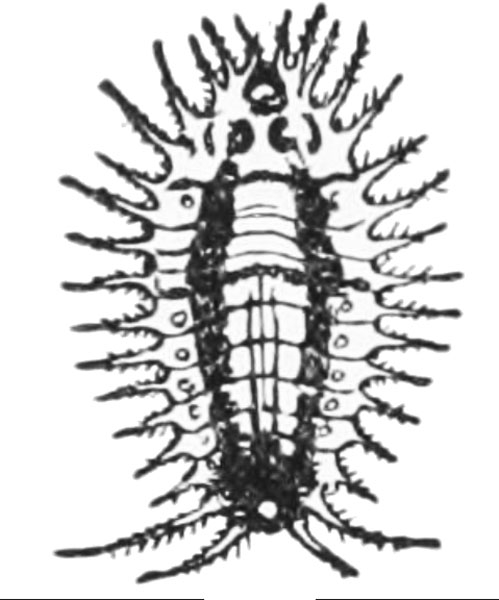
Adult—Whitefringed beetles are a conundrum to describe because there is much variation in the population, and a number of species may be involved. Because no male whitefringed beetles have been found in North America, it is impossible to test via cross-breeding whether the named species are identical. Adults are light to dark gray or brown, usually with a light band along the outer margins (the white fringe) and two pale lines or blotches along each side of the thorax and head. Beetles are about 1/2 inch long (Figure 26A).
Egg—The white, oval eggs, which are slightly less than 1/16 inch long, are laid in small masses in cracks just below the soil surface (Figure 26B). They are covered by a sticky secretion that soon hardens (moisture aids in their hatching). Eggs become pale yellow before hatching.
Larva—The slightly curved, yellowish-white grubs are legless, have a light-brown head, and measure up to 1/2 inch long (Figure 26C). Larvae may be found year-round in the soil.
Pupa—About 1/2 inch long, the white pupae gradually darken as they mature in the soil.
Biology
Distribution—Native to South America, whitefringed beetles were first reported in the United States in 1936 as pests of peanuts in Florida. They now occur from Florida to New Jersey and westward to Missouri and eastern Texas. They were first found in North Carolina in 1942 and now occur in scattered localities in 48 counties throughout the state.
Host Plants—Whitefringed beetles infest at least 385 plant species. Plants with taproots and smooth, broad leaves are most commonly damaged. Besides sweetpotato, important host plants include tobacco, peanut, corn, potato, soybean, velvet bean, strawberry, okra, cowpea, melon, bean, cotton, cauliflower, cabbage, cocklebur, and aster. Small grains can also be infested, though their fibrous root systems are more tolerant of damage.
Damage—Whitefringed beetles are relatively innocuous foliage feeders that leave notches on outer edges of leaves. The larvae, however, are particularly destructive to taproots and underground stems. On sweetpotatoes, whitefringed beetle damage consists of roughened holes and characteristic surface channels with rough ridges.
Life History—Whitefringed beetles usually overwinter as grubs, although eggs may survive the winter in protected locations. After feeding on sweetpotato roots, the grubs burrow 2 to 6 inches into the soil and pupate for 13 days. By early July, most larvae have matured and entered the pupal stage. Adults emerge mainly in July and early August. Females produce eggs without mating. They usually deposit eggs in clusters of 15 or 20 in the soil around the bases of host plants. Egg masses are held together by a sticky substance that hardens upon drying. Eggs are usually laid on or just below the surface of the soil. The number of eggs laid (600 to 700) depends on the type of host upon which the adults are feeding. Unless they overwinter, the eggs hatch 17 days after oviposition. The newly emerged larvae infest the roots of host plants until the onset of cold weather. One generation occurs each year. Whitefringed beetles are flightless but may crawl up to three quarters of a mile. There is one generation per year, although some grubs may not pupate until their second spring.
Control
Summer legumes may foster large whitefringed beetle populations. Grasses, including corn and small grains, are poor food for adults. Therefore, in known problem fields, rotate sweetpotatoes with grass crops to reduce whitefringed beetle populations.
In the past, quarantines were used to prevent the movement of infested soil and plant materials. Avoid planting in problem fields or control broadleaved weeds, use tolerant varieties, practice clean cultivation, and apply granular insecticide mid-season or spray foliar insecticides when adults are active. For the most up-to-date control information, consult your local N.C. Cooperative Extension center.
Yellowstriped Armyworm
Spodoptera ornithogalli (Guenée), Noctuidae, LEPIDOPTERA
Description
Adult—The yellowstriped armyworm moth has dark forewings with white and brown markings and white hind wings (Figure 27A). The wingspan ranges from 1 1/4 to 1 1/2 inches.
Egg—Almost 1/32 inch in diameter, the ribbed, greenish egg gradually becomes pale-pink to brown before hatching. The egg mass is covered with scales from the moth’s body.
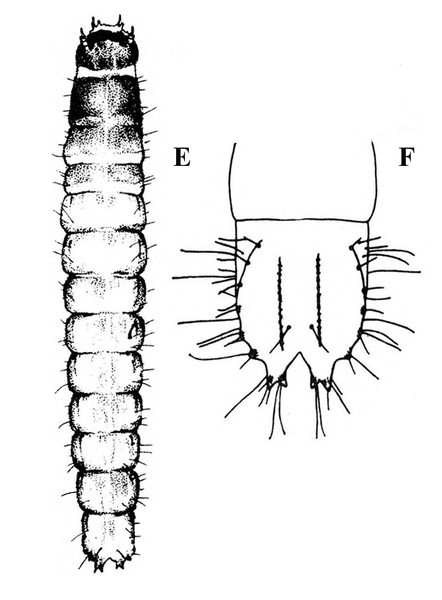
Larva—The smooth-skinned, pale-gray to jet-black caterpillar has a yellowish-orange stripe along each side and a pair of black triangular spots on the top of most segments (Figure 27B). Its head capsule is brown with black markings and a white, inverted V (Figure 27C). The sixth larval instar may be as long as 1 3/4 inches.
Pupa—The brownish pupa is about 3/4 inch long and 3/16 inch wide (Figure 27D).
Biology
Distribution—The yellowstriped armyworm occurs from New York southward into Mexico, westward to the Rocky Mountains, and in some areas of California and the West Indies. In the United States, it is most common and injurious in the southern states. In North Carolina, this caterpillar is observed annually in field and vegetable crops.
Host Plants—The yellowstriped armyworm is a general feeder. Besides sweetpotato, some of its hosts include alfalfa, asparagus, bean, beet, cabbage, clover, corn, cotton, cucumber, grape, grass, jimsonweed, morning glory, onion, pea, peach, peanut, tobacco, tomato, turnip, wheat, watermelon, and wild onion.
Damage—This foliage-feeding caterpillar is sporadically injurious to young crop stands. Defoliation of young plants can be a problem.
Life History-–Yellowstriped armyworms overwinter as pupae in the soil. In southern states, moth emergence begins in early April and continues into May. After mating, females deposit egg masses on foliage, trees, or buildings. About six days later, the eggs hatch, and the larvae begin feeding. Although these caterpillars appear by late spring in the South, they may not appear before July in northern and midwestern states. Larvae feed during the day on tender foliage over a three-week period. Mature sixth instars burrow into the soil and change into pupae. Two weeks later, the second generation of moths emerges. In southern states, including North Carolina, three to four generations occur each year.
Control
Yellowstriped armyworms seldom require control in North Carolina. Since large larvae are difficult to control with insecticides, early detection is important in suppressing populations to levels that don’t cause significant economic losses. For chemical control recommendations, consult the North Carolina Agricultural Chemicals Manual.
Other Resources
General
Harris, Jr., E. D. Control Sweet Potato Insects. Bulletin 668. University of Georgia Cooperative Extension, 1971.
Chalfant, R. B., S. A. Harmon, and L. Stacey. “Chemical Control of the Sweet Potato Flea Beetle and Southern Potato Wireworm on Sweet Potatoes in Georgia.” Journal of the Georgia Entomological Society 14 (1979): 354–58.
Covington, H. M., et al. Growing and Marketing Quality Sweet Potatoes. Circular 353. North Carolina Agricultural Extension Service, 1975.
Cuthbert, E. R. Insects Affecting Sweet Potatoes. Agriculture Handbook 329. USDA, 1967.
Sanderson, E. D. “Sweet Potato Insects.” Maryland Agricultural Experiment Station Bulletin 59 (1899): 129–68.
Smith, R. C. “Sweet Potato Insects in Kansas.” Kansas State Horticultural Society Biennial Report 50 (1950): 1–110.
Walsh, E. D., and C. V. Riley. “Insects Infesting the Sweet Potato.” American Entomologist 1 (1869): 234–38.
Lesser Fruit Flies
Demerec, M., ed. Biology of Drosophila. New York: Hafner Publishing Company, 1965.
Demerec, M., and R. P. Kaufmann. Drosophila Guide. 8th edition. Washington, DC: The Carnegie Institute of Washington, 1967.
Evans, M. “Welcome Advice—3. Fruit Flies (Drosophila spp.).” Food Manufacture 50, no. 2 (1975): 44.
Mason, H. O., and H. E. Dorst. Controlling Drosophila Flies on Tomatoes Grown for Canning. Farmers' Bulletin 2189. USDA, 1962.
Sturtevant, A. H. The North American Species of Drosophila. Publication No. 301. Washington, DC: The Carnegie Institution of Washington, 1921.
Wheeler, M. R. “Taxonomic and Distributional Studies of Nearctic and Neotropical Drosophilidae.” In Studies in the Genetics of Drosophila. IX, Articles on Genetics, Taxonomy, Cytology, and Radiation, Volume 9 (University of Texas Publication 5721), 70–114. Austin, TX: University of Texas, 1957.
Southern Armyworm
Aziz, S. A. “Toxicity of Certain Insecticide Standards Against the Southern Armyworm.” Journal of Economic Entomology 66 (1973): 68–70.
Chittenden, F. H., and H. M. Russell. “The Semi-Tropical Armyworm.” USDA Bureau of Entomology Bulletin 66 (1909): 53–70.
Crumb, S. E. The Semitropical Armyworm, Prodenia eridania Cram.” In Tobacco Cutworms (Technical Bulletin No. 88), 143–145. Washington, DC: U.S. Department of Agriculture, 1929.
Habeck, D. H. “Selective Feeding on Sweet Potato Varieties by Southern Armyworm.” Florida Entomology 59, no. 4 (1976): 396.
Hitchings, D. L. “Bacillus thuringiensis: A Reproductive Inhibitor for the Southern Armyworm.” Journal of Economic Entomology 60 (1967): 596–97.
Redfern, R. E. “Instars of Southern Armyworm Determined by Measurement of Head Capsule.” Journal of Economic Entomology 60 (1967): 614–15.
Spring Rose Beetle
Brimley, C. S. “Strigoderma Burmeister.” In The Insects of North Carolina, 205. Raleigh, NC: North Carolina Department of Agriculture, Division of Entomology, 1938.
Feakin, S. D. Strigoderma arboricola. In Pest Control in Groundnuts, 115–16. London: Centre for Overseas Pest Research, 1973.
Grayson, J. M. “Life History and Habits of Strigoderma arboricola.” Journal of Economic Entomology 39 (1959): 163–67.
Hayes, W. P. “Strigoderma arboricola Fab.—Its Life Cycle.” The Canadian Entomologist 53 (1921): 121–25.
Hoffman, C. H. “Additional Data on the Biology and Ecology of Strigoderma arboricola Fab. (Scarabaeidae—Coleoptera).” Brooklyn Entomological Society Bulletin 31 (1936): 108–10.
Miller, I. I. “A White Grub Injuring Peanuts in Eastern Virginia (Strigoderma arboricola (Fab).” Journal of Economic Entomology 36 (1943): 113–14.
Ritcher, P. O. The Anomalini of Eastern North America with Descriptions of the Larvae and a Key to Species (Coleoptera: Scarabaeidae). Bulletin 442. Kentucky Agricultural Experiment Station, 1943.
Ritcher, P. O. “Note on Strigoderma arboricola (Fab).” In Rutelinae of Eastern North America (Bulletin 471), 13. Kentucky Agricultural Experiment Station, 1945.
Sweetpotato Flea Beetle
Smith, J. B. “The Sweetpotato Flea-Beetle, Chaetocnema confinis Lec.” In Insects Injurious to Sweet Potatoes in New Jersey (Bulletin 229), 4–7. New Jersey Agricultural Experiment Station, 1910.
Sweetpotato Hornworm
Dyar, H. G. 1895. “Preparatory Stages of Phlegethontius cingulata.” Entomological News 6 (1895): 95–97.
Edwards, W. H. “Horn-Worms Which Defoliate Sweet Potato Vines.” Journal of the Jamaica Agricultural Society 41 (1937): 515.
Forbes, W. T. M. “A Structural Study of the Caterpillars. II. The Sphingidae.” Annals of the Entomology Society of America 4 (1911): 261–79.
Watson, J. R. “Herse cingulatus Fab. as an Armyworm.” The Florida Entomologist 27 (1944): 58.
Sweetpotato Weevil
Cockerham, K. L., et al. The Sweetpotato Weevil. Technical Bulletin 483. Louisiana Agricultural Experiment Station, 1954.
Reinhard, H. T. The Sweet Potato Weevil, Cylas formicarius Fabr., (Curculionidae). Bulletin 308. Texas Agricultural Experiment Station, 1923.
USDA. The Sweetpotato Weevil—How to Control It. Leaflet 431. USDA, 1960.
Sweetpotato Whitefly
Haviland, D. R., and J. T. Trumble. “Sweetpotato Whitefly (Silverleaf Whitefly).” In Pest Management Guidelines: Potato. Publication 3463. University of California Agriculture and Natural Resources, UC IPM, 2019.
McAuslane, H. J., et al. Common Name: Sweetpotato Whitefly B Biotype or Silverleaf Whitefly, Scientific Name: Bemisia tabaci (Gennadius) or Bemisia argentifolii Bellows & Perring (Insecta: Hemiptera: Aleyrodidae). Featured Creatures. Publication EENY–129. Entomology and Nematology, FDACS/DPI, EDIS, University of Florida, 2009.
Valverde, R. A., et al. “Whitefly Transmission of Sweet Potato Viruses.” Virus Research 100, no. 1 (2004): 123–28.
Tortoise Beetles
Barber, H. S. “A Review of North American Tortoise Beetles.” Entomological Society of Washington Proceedings 18 (1917): 113–27.
Chittenden, F. H. Insects Injurious to Vegetables. New York: Orange Judd Company, 1909.
Chittenden, F. H. “The Argus Tortoise Beetle, Chelymorpha cassidea Fab., (Chrysomelidae).” Journal of Agricultural Research 27 (1924): 43–51.
Smith, J. B. “The Gold-Bugs or Tortoise Beetles.” In Insects Injurious to Sweet Potatoes in New Jersey (Bulletin 229), 8–12. New Jersey Agricultural Experiment Station, 1910.
Woodruff, R. E. The Tortoise Beetles of Florida IV, Metriona bicolor (Fab) (Coleoptera: Chrysomelidae). Entomology Circular 164. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, 1976.
Whitefringed Beetles
Dixon, W. N. Common Name: Whitefringed Beetles, Scientific Name: Naupactus (=Graphognathus) spp. (Insecta: Coleoptera: Curculionidae). Featured Creatures. Publication Number: EENY–294. Entomology and Nematology, FDACS/DPI, EDIS, University of Florida, 2008.
O'Sullivan, J. Whitefringed Beetle, Scientific name: Naupactus leucoloma (Boheman); Naupactus minor (Buchanan); Naupactus peregrinus (Buchanan). In Sweetpotato DiagNotes: A Diagnostic Key and Information Tool for Sweetpotato Problems, The University of Queensland, n.d.
Roberts, R. A. “Whitefringed Beetle.” In Insects, The Yearbook of Agriculture 1952, 608–11. Washington, DC: U.S. Department of Agriculture, 1952.
USDA. Controlling Whitefringed Beetles. Leaflet 550. USDA, 1972.
Van Driesche, R. G., J. H. LaForest, C.T Bargeron, R. C. Reardon, and M. Herlihy. “Whitefringed Beetles (Naupactus spp.) (Coleoptera: Curculionidae).” In Forest Pest Insects in North America: A Photographic Guide. UMass Amherst, USDA Forest Service, Center for Invasive Species and Ecosystem Health, and The University of Georgia, 2013.
Young, H. C., B. A. App, J. B. Gill, and H. S. Hollingworth. Whitefringed Beetles and How to Combat Them. Circular 850. USDA, 1950.
Yellowstriped Armyworm
Crumb, S. E. “The Army Worms.” Bulletin of the Brooklyn Entomological Society 22 (1927): 41–55.
Walkden, H. H. Cutworms, Armyworms, and Related Species Attacking Cereal and Forage Crops in the Central Great Plains. Circular 849. USDA, 1950.
Whelan, D. B. A Key to the Nebraska Cutworms and Armyworms That Attack Corn. Research Bulletin 81. Nebraska Agricultural Experiment Station, 1935.
Publication date: June 14, 2024
AG-295
Other Publications in Insect and Related Pests of Vegetables
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.