Outline
Seeds
Germination
Seed Dormancy
Techniques to Break Dormancy
Seed Scarification
Seed Stratification
Growing Plants from Seed
Media
Sterilizing Containers
Sowing Seeds
Water and Light
Transplanting Seedlings
Cuttings
Root Cuttings
Stem Cuttings
Cane Cuttings
Leaf Bud Cuttings
Leaf Cuttings
Asexual Propagation- Other Methods
Layering
Separation and Division
Budding and Grafting
Micropropagation
Objectives
This chapter teaches people to:
- Understand the basic principles of sexual and asexual propagation.
- Be familiar with common methods of sexually and asexually propagating plants.
- Know the recommended timing for propagating plants.
Introduction
Plant propagation is the process of producing a new plant from an existing one. It is both art and science requiring knowledge, skill, manual dexterity, and experience for success.
To understand the science of why, when, and how to propagate requires basic knowledge of plant growth and development, plant anatomy and morphology, and plant physiology.
There are two general types of propagation: sexual and asexual. Sexual propagation is the reproduction of plants by seeds. The genetic material of two parents is combined by pollination and fertilization to create offspring that are different from each parent. There are several advantages of sexual propagation:
- It may be quicker and more economical than asexual propagation.
- It may result in new cultivars and vigorous hybrids.
- For some plants, it may be the only means of propagation.
- It provides a way to avoid transmission of particular diseases, such as viruses.
- It maintains genetic variation, which increases the potential for plants to adapt to environmental pressures.
Asexual propagation, sometimes referred to as vegetative propagation, involves taking vegetative parts of a plant (stems, roots, and/or leaves) and causing them to regenerate into a new plant or, in some cases, several plants. With few exceptions, the resulting plant is genetically identical to the parent plant. The major types of asexual propagation are cuttings, layering, division, separation, grafting, budding, and micropropagation. Advantages of asexual propagation include:
- It may be easier and faster than sexual propagation for some species.
- It may be the only way to perpetuate particular cultivars.
- It maintains the juvenile or adult characteristics of certain cultivars.
- It allows propagation of special types of growth, such as weeping or pendulous forms.
- It may more quickly result in a large plant (compared to one propagated by seed).
A form of asexual reproduction in plants, in which multicellular structures become detached from the parent plant and develop into new individuals that are genetically identical to the parent plant.
Sexual Propagation
Seeds
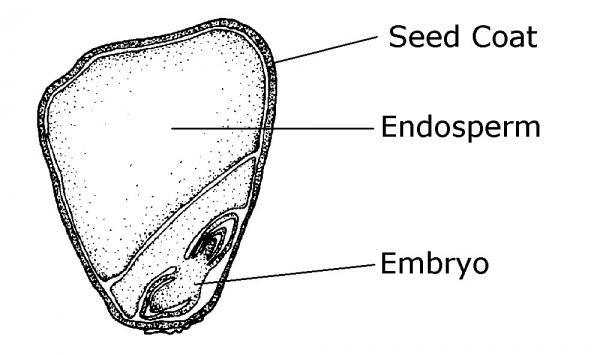
Most seeds are composed of three major parts: embryo, endosperm (food storage) tissue, and a seed coat (protective tissue) (Figure 13–1). The embryo is a miniature plant in a resting (dormant) state. Most seeds contain a built-in food supply called the endosperm. The protective outer covering of a seed is called the seed coat. It protects seeds from mechanical injury and from diseases and insects. Also, the seed coat usually prevents water from entering the seed until time to germinate. The seed coat in many cases allows seeds to be stored for extended periods. The seed leaves, cotyledons, differ in shape from the true leaves. Monocots (such as corn) produce only one cotyledon; dicots (like beans) produce two cotyledons (Figure 13–2). Some gymnosperms, like pines, have many cotyledons.
To obtain vigorous plants from seeds, start with high-quality seeds from a reliable source. Select cultivars that provide the desired size, color, and growth habit. Choose cultivars adapted to your area. Many vegetable and flower cultivars are hybrids that may cost more than open-pollinated types, but they usually have more vigor, more uniformity, and better growth than nonhybrids.
Purchase only enough seed for one year because the likelihood of germination decreases with age. The seed packet label usually indicates essential information about the cultivar or species, such as the year in which the seeds were packaged, the germination percentage, and whether the seeds have received any chemical treatment.
If seeds are obtained well ahead of the actual sowing date (or are surplus seeds), store them in a cool, dry place. Laminated or foil packages help ensure dry storage. Paper packets are best kept in tightly sealed containers and maintained around 40°F in low humidity. A good storage location would be an airtight jar in the refrigerator. Gardeners can save money and cultivate a rewarding hobby by saving seeds from plants in their own gardens. Seeds that have been produced through insect, animal or wind, or other natural pollination methods are known as open-pollinated. Open-pollination can increase biodiversity, and plants may display different characteristics than the parent plants. This is especially true when saving seed from hybrids.
Table 13–1. Germination information for selected plants.
| Plant | Approximate Time to Sow Before Last Frost (weeks) | Time Seeds Take to Germinate (days) | Temperature (°F) | Light/Dark Requirement |
|---|---|---|---|---|
| Ageratum | 8 | 5–10 | 70 | Light |
| Alyssum | 8 | 5–10 | 70 | Either |
| Aster | 6 | 5–10 | 70 | Either |
| Balsam | 6 | 5–10 | 70 | Either |
| Begonia | 12 or more | 10–15 | 70 | Light |
| Broccoli | 8 | 5–10 | 70 | Either |
| Browallia | 12 or more | 15–20 | 70 | Light |
| Cabbage | 8 | 5–10 | 70 | Either |
| Cauliflower | 8 | 5–10 | 70 | Either |
| Celosia | 8 | 5–10 | 70 | Either |
| Centaurea | 6 | 5–10 | 65 | Dark |
| Coleus | 8 | 5–10 | 65 | Light |
| Cosmos | 4 or less | 5–10 | 70 | Either |
| Cucumber | 4 or less | 5–10 | 85 | Either |
| Dahlia | 8 | 5–10 | 70 | Either |
| Dianthus | 10 | 5–10 | 70 | Either |
| Eggplant | 8 | 5–10 | 70 | Either |
| Geranium | 12 or more | 10–20 | 70 | Light |
| Impatiens | 10 | 15–20 | 70 | Light |
| Larkspur | 12 or more | 5–10 | 55 | Dark |
| Lettuce | 8 | 5–10 | 70 | Light |
| Marigold | 6 | 5–10 | 70 | Either |
| Muskmelon | 4 or less | 5–10 | 85 | Either |
| Nicotiana | 8 | 10–15 | 70 | Light |
| Pansy (Viola) | 12 or more | 5–10 | 65 | Dark |
| Pepper | 8 | 5–10 | 80 | Either |
| Petunia | 10 | 5–10 | 70 | Light |
| Phlox | 8 | 5–10 | 65 | Dark |
| Portulaca | 10 | 5–10 | 70 | Dark |
| Snapdragon | 10 | 5–10 | 65 | Light |
| Squash | 4 or less | 5–10 | 85 | Either |
| Tomato | 6 | 5–10 | 80 | Either |
| Verbena | 10 | 15–20 | 65 | Dark |
| Vinca | 12 or more | 10–15 | 70 | Either |
| Watermelon | 4 or less | 5–10 | 85 | Either |
| Zinnia | 6 | 5–10 | 70 | Either |
Germination
Germination is the resumption of active embryo growth after a dormant period. Three conditions must be satisfied for a seed to germinate:
- The seed must be viable; that is, the embryo must be alive and capable of germination.
- Internal conditions of the seed must be favorable for germination; that is, any physical, chemical, or physiological barriers to germination must have disappeared or must have been removed by the propagator.
- The seed must be subjected to appropriate environmental conditions, including water (moisture), proper temperature, oxygen, and, for some species, light (Table 13–1).
The first step in germination is absorption of water. An adequate, continuous supply of moisture is important to ensure germination. Once germination has begun, a dry period can kill the embryo.
Light can stimulate or inhibit seed germination of some species. Plants that require light for germination include ageratum, begonia, browallia, impatiens, lettuce, and petunia. Other plants germinate best in the dark. These include calendula, centaurea, phlox, and verbena. Some plants germinate in either light or dark. Seed catalogs and seed packets often list germination and cultural information for particular plants. When sowing light-requiring seeds, sow them on the soil surface. Supplemental light can be provided by fluorescent fixtures suspended 6 to 12 inches above the soil surface for 16 hours a day.
Respiration in dormant seeds is low, but they do require some oxygen. Respiration rate increases during germination. The medium in which the seeds are sown should be loose and well aerated. If the oxygen supply during germination is limited or reduced, germination can be severely retarded or inhibited.
Temperature affects the germination percentage and the rate (speed) of germination. Some seeds germinate over a wide range of temperatures; others have a narrow range. Many species have minimum, maximum, and optimum temperatures at which they germinate. For example, tomato seeds have a minimum germination of 50°F, a maximum of 95°F, and an optimum germination temperature of 80°F. When germination temperatures are listed, they are usually optimum temperatures. For most plants, 65 to 75°F is best.
Seed Dormancy
Viable seeds that do not germinate are dormant. Dormancy can be regulated by the environment or by the seed itself. If a seed is not exposed to sufficient moisture, proper temperature, oxygen, or for some species, light, the seed will not germinate. In this case, the seed's dormancy is caused by unfavorable environmental conditions.
Some seeds may not germinate because of some inhibitory factor of the seed itself. This kind of dormancy consists of two general types: (a) seed coat (or external) dormancy and (b) internal (endogenous) dormancy. A seed can also exhibit both kinds of dormancy.
Techniques to Break Dormancy
Seed Scarification
External dormancy results when a seed's hard seed coat is impervious to water and gases. The seed will not germinate until the seed coat is altered physically. Any process of breaking, scratching, or mechanically altering the seed coat to make it permeable to water and gases is known as scarification. In nature this may occur during the winter, when freezing temperatures crack the seed coat or while microbial activities modify the seed coat as the seed lies in the soil. Scarification may also occur as the seed passes through the digestive tract of an animal.
Scarification can be forced, rather than waiting for nature to alter the seed coats. Commercial growers scarify seeds by soaking them in concentrated sulfuric acid. Seeds are placed in a glass container, covered with sulfuric acid, gently stirred, and allowed to soak for 10 minutes to several hours, depending on the species.
Reference books give appropriate concentrations and durations. When the seed coat has been modified (thinned), the seeds are removed, washed, and sown. Sulfuric acid can, however, be very dangerous for an inexperienced individual and should be used with extreme caution. Vinegar is safer and can be used for some species; the technique is the same as with sulfuric acid.
With mechanical scarification, seeds are filed with a metal file, rubbed with sandpaper, or cracked gently with a hammer to weaken (break) the seed coat. Another method is hot water scarification. Bring water to a boil (212°F), remove the pot from the stove, and place the seeds into the water. Soak the seeds until the water cools; then remove them and let them dry.
Seed Stratification
The second type of imposed dormancy, internal dormancy, is regulated by the inner seed tissues. This dormancy prevents seeds of many species from germinating when environmental conditions are not favorable for survival of the seedlings. There are several different types of internal dormancy. "Shallow" dormancy, displayed by many vegetable seeds, simply disappears with dry storage. No special treatment is necessary. However, other types require a particular duration of moist-chilling or moist-warming periods, or both.
Cold stratification (moist-chilling) involves mixing seeds with an equal volume of a moist medium (sand or peat, for example) in a closed container and storing them in a refrigerator. Periodically, check to see that the medium is moist but not wet. The length of time required to break (remove) dormancy varies by species; check reference books for recommended times. This type of dormancy may be satisfied naturally if seeds are sown outdoors in the fall. Warm stratification is similar except temperatures are maintained at 68 to 86°F depending on the species.
Seeds of some species exhibit double dormancy. This is a combination of two types of dormancy, such as external and internal dormancy. To achieve germination with seeds having both external and internal dormancy, the seeds must first be scarified and then stratified for the appropriate length of time. If the treatments are administered in reverse order, the seeds will not germinate. After completing these treatments, plant the seeds under the proper environmental conditions for germination.
Growing Plants from Seeds
Media
A wide range of media can be used to germinate seeds. With experience, you will learn to determine what works best for you. The germinating medium should be fine and uniform yet well aerated and loose. It should be free of insects, disease organisms, nematodes, weeds, and weed seeds. It should also be of low fertility and capable of holding moisture but be well drained. Purchase commercial potting media containing fine-particle pine bark, sphagnum peat moss, and perlite, or prepare a combination of equal parts (by volume) of these materials. Do not use garden (mineral) soil to start seedlings; it is not sterile, it is too heavy, and it does not drain well. Soil mixes have little fertility, so seedlings must be watered with a dilute fertilizer solution soon after germination and emergence.
Containers
Plastic cell packs can be purchased or reused if sterilized. In this system, each cell holds a single plant. This method reduces the risk of root injury when transplanting. Peat pellets, peat pots, or expanded foam cubes can also be used for producing seedlings. Resourceful gardeners often use cottage cheese containers, the bottoms of milk cartons, bleach containers, or pie pans. Just make certain that adequate drainage holes are made in the bottoms of the containers and that the containers are sterile.
Sterilizing Containers
The importance of using sterile medium and containers cannot be overemphasized. Before using the containers, wash them to remove any debris, immerse them in a fresh solution of one part chlorine bleach to nine parts water for five minutes, and allow them to dry.
Sowing Seeds
Seedlings are often started indoors 4 to 12 weeks before the last spring frost (Table 13–1). A common mistake is to sow the seeds too early and then attempt to hold the seedlings under poor environmental conditions. This usually results in tall, weak, spindly plants that do not perform well in the garden. The following paragraphs give general guidelines for sowing seeds for transplants. Nevertheless, it is important to refer to the instructions on the seed packet for more specific information.
When sowing seeds, fill the container to within 3⁄4-inch of the top with moistened growing medium. For very small seeds, use a fine, screened medium such as a layer of fine vermiculite for the top 1⁄4-inch. Firm the medium at the corners and edges with your fingers or a block of wood to provide a smooth and level surface.
For medium and large seeds, make furrows 1 to 2 inches apart and 1⁄8-inch to 1⁄4-inch deep across the surface of the planting medium. Sowing in rows improves light and air movement. If damping-off disease occurs, there is less chance of it spreading. Seedlings in rows are easier to label and handle at transplanting time than those that have resulted from broadcasting seeds. Sow the seeds thinly and uniformly in the rows by gently tapping the packet of seed. Cover the seeds lightly; a suitable planting depth is usually about two to four times the minimum diameter of the seeds.
Extremely fine seeds such as carrot, petunia, and snapdragon should not be covered, but simply dusted on the surface of the germinating medium and watered with a fine mist. If these seeds are broadcast, strive for a uniform stand by sowing half the seeds in one direction, then sowing the remaining seeds in the other direction.
Large seeds are frequently sown directly in a small container or cell pack, which eliminates the need for early transplanting. Usually, sow two or three seeds per cell. Later, thin them to allow only the most vigorous seedling per cell to grow.
Most garden stores and seed catalogs offer indoor and outdoor seed tapes. Seed tapes have precisely spaced seeds enclosed in an organic, water-soluble material. When planted, the tape dissolves and the seeds germinate normally. Seed tapes are convenient for extremely small, hard-to-handle seeds. Seed tapes allow uniform emergence, eliminate overcrowding, and permit sowing in perfectly straight rows. The tapes can be cut at any point for multiple row plantings, and thinning is rarely necessary. Tapes are more expensive per seed.
Water and Light
Moisten the planting medium thoroughly before planting. After seeding, spray with a fine mist or place the containers in a pan or tray that contains about 1 inch of warm water. Avoid splashing or excessive flooding, which might displace small seeds. When the planting mix is saturated, set the container aside to drain. The soil should be moist but not overly wet.
The seed flats should remain sufficiently moist during the germination period. Excessive moisture, however, can lead to damping-off or other disease or insect problems. Place the whole flat or pot into a clear plastic bag to maintain moisture. The plastic should be at least 1 inch above the soil. Keep the container out of direct sunlight; otherwise, the temperature may increase and injure the seeds. Many home gardeners cover their flats with panes of glass instead of using a plastic bag. Be sure to remove the plastic bag or glass cover when the first seedlings emerge.
After the seeds have germinated, move the flats to a well-lighted location; the temperature should be 65 to 70°F during the day and 55°F to 60°F at night. This prevents soft, leggy growth and minimizes disease problems. Some crops, of course, may grow best at different temperatures.
Seedlings must receive bright light after germination. Place them in a south-facing window. If a large, bright location is not available, place the seedlings under fluorescent lights. Use two 40-watt, cool-white fluorescent tubes or special plant growth lamps. Position the plants 6 inches below the light source and provide 16 hours of light daily. As the seedlings grow, the lights should be raised. A more detailed discussion of lighting is covered in chapter 18, "Plants Grown in Containers."
Transplanting Seedlings
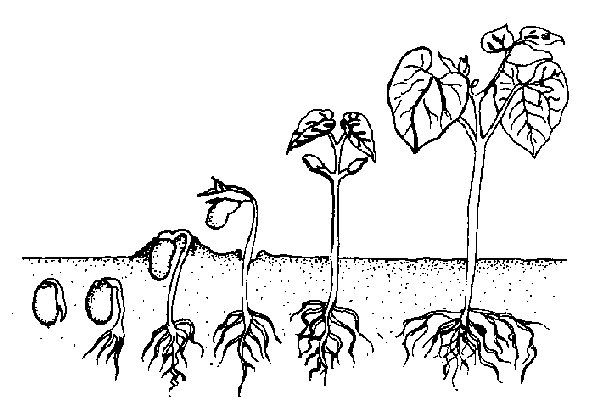
Plants not seeded individually must eventually be transplanted into their own containers as seedlings to give them proper growing space. A common mistake is to leave the seedlings in the flat too long. The ideal time to transplant young seedlings is when the first true leaves appear.
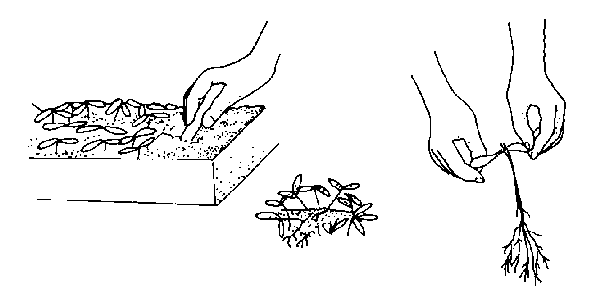
Dig up the small plants carefully with a knife or plant label. Let the group of seedlings fall apart and pick out individual plants. Gently ease them apart to avoid root injury in the process. Handle small seedlings by their leaves, not their delicate stems (Figure 13–3). Using a small tool or your finger, punch a hole in the medium. Plant a seedling at the same depth at which it was growing in the seed flat. Firm the soil and water gently. Keep newly transplanted seedlings in the shade for a few days, or place them under fluorescent lights. Locate them away from sources of direct heat. Continue watering and fertilizing as in the seed flats.
Containers for seedlings should be economical, durable, and make efficient use of available space. Individual pots or plastic cell packs can be used. Another possibility is compressed peat pellets, which expand to form compact individual pots when soaked in water.
They waste no space, do not fall apart as easily as peat pots, and can be set out directly in the garden. If you wish to avoid transplanting seedlings altogether, compressed peat pellets are excellent for direct sowing.
When setting plants outdoors that were grown in peat pots, be sure to break the sides of the pot and to cover the pot completely. If the top edge of the peat pot extends above the soil level, it may act as a wick and draw water away from the soil in the pot. Tear off the top lip of the pot and plant flush with the soil surface.
Examples of Seed Treatments
Camellia—Collect and plant in the fall before the seed coat hardens. If seeds are dry, soak them in warm water for 24 hours before planting. Some people pre-chill the seeds until radicle emergence and then plant the sprouted seeds.
Crabapple—Collect fruits as they begin to soften and when the seeds are brown. Remove the fruit pulp. Provide one to four months of cold-moist stratification. Seeds will germinate in 30 to 60 days.
Dogwood—Collect fruits (drupes) when they are red and when seeds are mature; if collection is delayed too long, birds may eat the fruit. Remove the pulp, clean, and air dry, then moist-chill them in a refrigerator for three to four months. Seeds can be planted in the fall, but they will not germinate until spring.
Goldenrain tree—Collect fruits when capsules turn brown but before they open. Extract seeds, dry, and store. Seed coats are very hard, and seeds will require scarification before germination.
Holly—Germinating holly seeds can be very difficult and extremely slow. It may take two to three years because of the holly's hard seed coat and an immature (rudimentary) embryo.
Maple—Variation in dormancy exists with different species of maples. Spring-maturing seeds of species such as red and silver maple should be collected immediately when mature, not permitted to dry, and sown immediately. For seeds of other maple species that mature in the fall, such as southern sugar maple, stratification for 90 to 120 days is necessary.
Oak—Acorns of white oak do not become dormant. When planted in the fall, roots will emerge during winter; shoots will emerge in the spring. On the other hand, acorns of black oak germinate best if stratified for one to three months (if not planted in the fall). Acorns of red oaks should be planted in the fall or stratified for one to three months. Check references for individual species.
Redbud—Germination is inhibited by an impermeable seed coat and embryo dormancy. Soak for 30 minutes in concentrated sulfuric acid or vinegar, and then follow up with three months of cold stratification.
Southern magnolia—Remove the fleshy pulp from around the seeds. Moist pre-chilling for two to four months is needed unless planted in the fall.
Asexual Propagation Cuttings
Asexual propagation is the process of taking vegetative pieces of a desirable plant and reproducing new plants from these tissues. Asexual propagation permits cloning of plants, meaning the resulting plants are genetically identical to the parent plant. The major methods of asexual propagation are cuttings, layering, division, separation, budding, grafting, and micropropagation (tissue culture).
CUTTINGS
Propagation by cuttings involves rooting a severed piece of the parent plant or, in some cases, producing new plants from severed pieces of tissue (leaf cuttings). A greenhouse is not necessary for successful propagation by cuttings.
If rooting only a few cuttings, you can use a flowerpot or small flat (Figure 13–4). Maintain high humidity by covering the cuttings with a bottomless milk jug or by placing the container into a clear plastic bag. Cuttings can also be placed in plastic trays covered with clear plastic stretched over a wire frame.
Containers must have holes in the bottoms for drainage. The plastic helps keep the humidity high and reduces water loss from the plant. If a more elaborate structure is needed, construct a small hoop frame or use an intermittent mist system. NC State Extension publication AG-426, A Small Backyard Greenhouse for the Home Gardener, may be helpful.
The rooting medium should be sterile, low in fertility, well drained to provide sufficient aeration, and moisture-retentive so that watering does not have to be done too frequently. Materials commonly used are coarse sand, a mixture of one part peat and one or two parts perlite (by volume), or one part peat and one part sand (by volume). Various commercial potting media may also be used. Vermiculite by itself is not recommended because it packs and tends to hold too much moisture. Media should be watered well before use.
There are several different kinds of cuttings. Which type you use depends on the kind of plant and, often, the plant's growth stage.
Some plants can be propagated from only a leaf, but most plants produce only a few roots or simply decay. Because leaf cuttings do not include an axillary bud, they can be used only for plants that are capable of forming adventitious buds. Leaf cuttings are used almost exclusively for propagating some indoor plants.
Leaf Cuttings
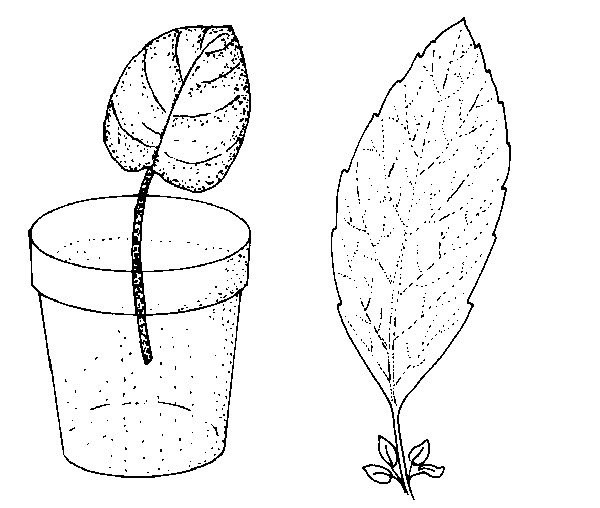
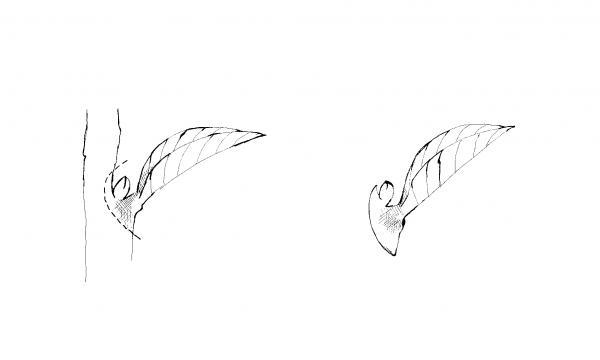
Leaf petiole—Remove a leaf and include up to 1½-inches of the petiole. Insert the lower end of the petiole into the medium (Figure 13–5). One or more new plants form at the base of the petiole. The leaf may be severed from the new plants—when they have their own roots—and then reused. Examples of plants that can be propagated by this method include African violet, peperomia, episcia, hoya, and sedum.
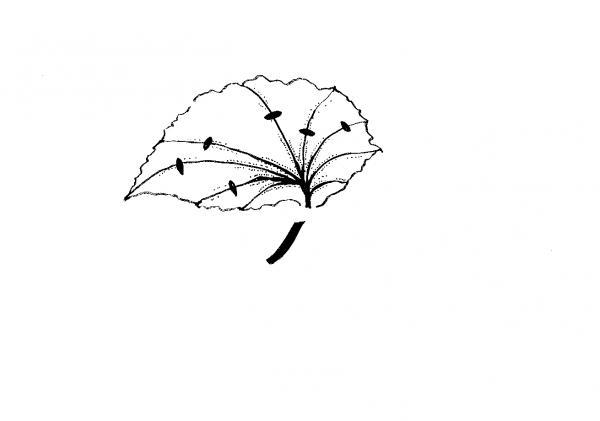
Leaf without a petiole—This method is used for plants with thick, fleshy leaves. The snake plant (Sansevieria trifasciata), a monocot, can be propagated by cutting the long leaves into 3-inch to 4-inch pieces (Figure 13–6). Insert the cuttings vertically into the medium. African violets (dicot) can also be propagated this way. Cut a leaf from a plant and remove the petiole. Insert the leaf vertically into the medium, making sure that the midvein is buried in the rooting medium. New plants form from the midvein.
Split-vein—Detach a leaf from the plant and remove the petiole. Make cuts on several prominent veins on the underside of the leaf (Figure 13–7). Lay the cutting, lower side down, on the medium. New plants form at each cut. If the leaf curls up, hold it in place by covering the margins with rooting medium. A variation of this method is to cut the leaf into wedges so that each piece has a main vein.
Leaf-Bud Cuttings
Leaf-bud cuttings are used for many trailing vines and when space or cutting material is limited. Each node on a stem can be treated as a cutting. The cutting consists of a leaf blade, petiole, and a short piece of stem with an attached axillary bud. Place cuttings in the medium with the bud covered (to 1 inch) and the leaf exposed (Figure 13–8). A modified version of a leaf-bud cutting, referred to as a single node cutting, can be prepared simply by cutting the stem below and above the leaf petiole having a well-developed axillary bud. Examples of plants propagated this way include blackberry, camellia, clematis, devil's ivy, dracaena, grape ivy, heart-leaf philodendron, jade plant, mahonia, rhododendron, and rubber plant.
Cane Cuttings
A cane cutting is an easy way to propagate some overgrown, leggy houseplants such as dumbcane, corn plant, Chinese evergreen, and other plants with thick stems. Leafless stem sections (2 to 3 inches long) are cut from older stems. Each cane should contain one or two nodes (Figure 13–9). Lay the cutting horizontally on the medium, or insert it vertically with about half of the cutting below the surface of the medium, and leave a bud facing upward. Cane cuttings are usually potted when roots and new shoots appear.
Stem Cuttings
Propagation by stem cuttings is the most commonly used method for many woody ornamental plants. Typically, stem cuttings of tree species are more difficult to root successfully; however, cuttings from trees such as crape myrtles, some elms, and birches can be rooted.
Types of Stem Cuttings
The four main types of stem cuttings are herbaceous, softwood, semi-hardwood, and hardwood. These terms reflect the growth stage of the stock plant, which is one of the most important factors influencing whether cuttings produce roots. Calendar dates are useful only as guidelines. Refer to Table 13–2 for more information on optimum growth stages for rooting stem cuttings of various woody ornamentals.
Herbaceous cuttings are made from nonwoody, herbaceous plants such as coleus, chrysanthemums, and dahlia. A 3-inch to 5-inch piece of stem is cut from the parent plant. The leaves on the lower one-third to one-half of the stem are removed, and the cutting is placed in the rooting medium. A high percentage of the cuttings root, and they do so quickly.
Softwood cuttings are prepared from soft, succulent, new growth of woody plants, just as it begins to harden (mature). Shoots are suitable for making softwood cuttings when they can be snapped easily when bent and when they still have a gradation of leaf size (oldest leaves are mature whereas newest leaves are still small). For most woody plants this stage occurs in May, June, or July. The soft shoots are quite tender, and extra care must be taken to keep them from drying out. The extra effort pays off, though, because they root quickly.
Semi-hardwood cuttings are usually prepared from partially mature wood of the current season's growth, just after a flush of growth. This type of cutting normally is made from mid-July to early fall. The wood is reasonably firm and the leaves of mature size. Many broadleaf evergreen shrubs and some conifers are propagated by this method.
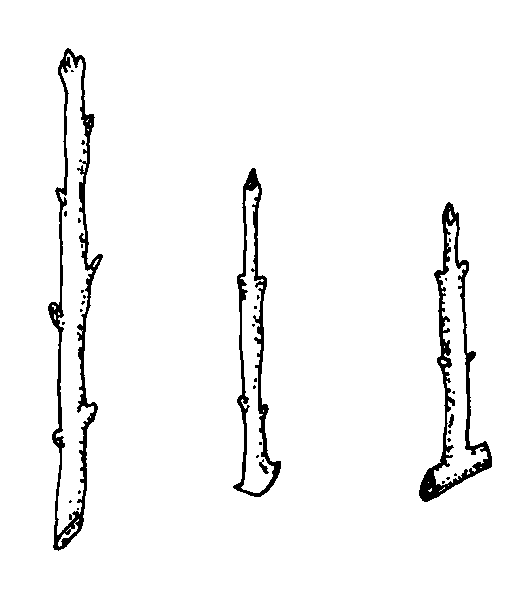
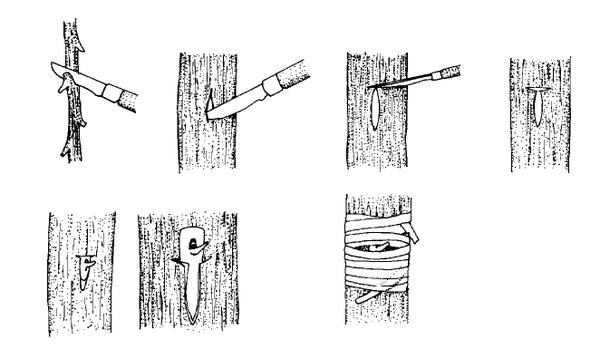
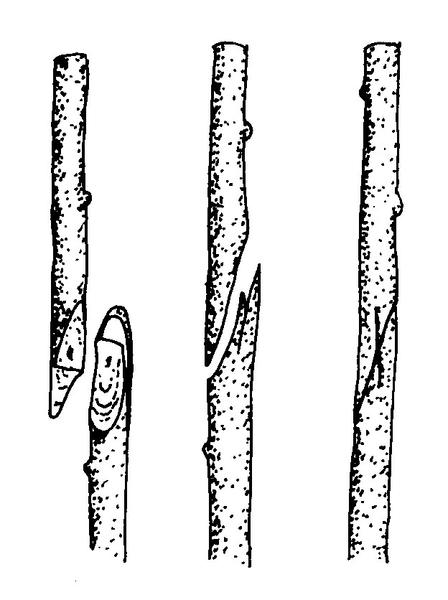
Hardwood cuttings are taken from dormant mature stems in late fall, winter, or early spring. Plants are generally fully dormant with no obvious signs of active growth. The wood is firm and does not bend easily. Hardwood cuttings are most often used for deciduous shrubs but can be used for many evergreens. Examples of plants propagated at the hardwood stage include fig, forsythia, grape, privet, and spirea. The three types of hardwood cuttings are straight, heel, and mallet (Figure 13–10). A straight cutting is the most commonly used stem cutting. For the heel cutting, a small section of older wood is included at the base of the cutting. For the mallet cutting, an entire section of older stem wood is included.
Propagating Ferns by Spores
Although ferns are propagated more easily by division, some gardeners like the challenge of raising ferns from spores. Spores are the fern's means of sexual propagation, equivalent to seeds. The following is one proven method for germinating small quantities of spores.
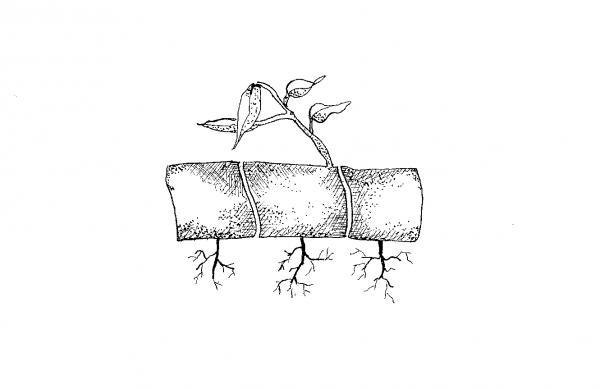
Place a solid, sterilized brick in a pan, add water to cover the brick, and bake at 250°F for 30 minutes. When the brick is wet throughout, remove it from the water and place a thin layer of moist soil and peat (1:1 by volume) on top of the brick. Dust spores on the medium. Cover with plastic (not touching the spores) and place the brick in a warm place with indirect light. It may take up to a month for the spores to germinate. Keep moist at all times.
A small, heart-shaped structure, about 1⁄8-inch across (called a prothallus) develops first from each spore, forming a light green mat. Mist lightly to maintain high surface moisture because sperm must be able to swim to the archegonia (female organ). After about three weeks, fertilization should have occurred.
About two months later, pull the mat apart with tweezers in 1⁄4-inch squares and space them 1⁄2-inch apart in a flat containing a 2-inch bottom layer of sand, a 1⁄4-inch layer of charcoal, and a top 2-inch layer of a mixture of potting soil and peat (1:1 by volume). Cover with plastic and keep moist. When fronds (fern "leaves") appear, transplant to small pots. Reduce humidity gradually until plants can survive in less humid conditions. Light exposure may be increased at this time.
| Common Name | Scientific Name | Type of Cuttinga |
|---|---|---|
| Abelia | Abelia spp. | SW, SH, HW |
| Arborvitae, American; Northern white-cedar | Thuja occidentalis | SH, HW |
| Arborvitae, Oriental | Platycladus orientalis | SW |
| Azalea (evergreen & semi-evergreen) | Rhododendron spp. | SH |
| Barberry, Japanese b | Berberis thunbergii | SH, HW |
| Barberry, Mentor | Berberis x mentorensis | SH |
| Barberry, Wintergreen | Berberis julianae | HW |
| Bayberry; Wax myrtle | Myrica spp. | SW |
| Boxwood, Common | Buxus sempervirens | SH, HW |
| Boxwood, Littleleaf | Buxus microphylla | SH, HW |
| Camellia | Camellia spp. | SH, HW |
| Ceanothus | Ceanothus spp. | SW, SH |
| Cedar | Cedrus spp. | SH, HW |
| Cedar, Eastern red | Juniperus virginiana | HW |
| Cotoneaster | Cotoneaster spp. | SW, SH |
| Cryptomeria, Japanese | Cryptomeria japonica | SW, SH, HW |
| Cypress, False | Chamaecyparis spp. | SH, HW |
| Cypress, Leyland | x Cuprocyparis leylandii | SW, HW |
| Daphne | Daphne spp. | SH |
| Eleagnus, Thorny; Silverthorn | Elaeagnus pungens | SH, HW |
| Euonymus b | Euonymus spp. | SH |
| Fir | Abies spp. | SW, HW |
| Gardenia, Cape jasmine | Gardenia jasminoides | SH, HW |
| Heath | Erica carnea | SW, SH, HW |
| Heather, Scotch | Calluna vulgaris | SH, HW |
| Hemlock | Tsuga spp. | SW, HW |
| Holly, American | Ilex opaca | SH, HW |
| Holly, Chinese | Ilex cornuta | SH, HW |
| Holly, English | Ilex aquifolium | SH, HW |
| Holly, Foster's | Ilex x attenuata 'Fosteri' | SH, HW |
| Holly, Japanese | llex crenata | SH, HW |
| Holly, Yaupon | Ilex vomitoria | SH |
| Ivy, English b | Hedera helix | SH, HW |
| Jasmine | Jasminum spp. | SW, SH, HW |
| Juniper, Chinese | Juniperus chinensis | SH, HW |
| Juniper, Creeping | Juniperus horizontalis | SH, HW |
| Juniper, Shore | Juniperus conferta | SH, HW |
| Loropetalum | Loropetalum chinense | SW |
| Magnolia | Magnolia spp. | SW, SH |
| Mahonia b | Mahonia spp. | SH, HW |
| Oleander | Nerium oleander | SH |
| Osmanthus, Holly; False holly | Osmanthus heterophyllus | SH, HW |
| Photinia | Photinia spp. | SH, HW |
| Pieris, Japanese; Japanese Andromeda | Pieris japonica | SW, SH |
| Pine | Pinus spp. | SW, HW |
| Pine, Mugo | Pinus mugo | SH |
| Pittosporum | Pittosporum tobira | SH |
| Podocarpus | Podocarpus spp. | SH |
| Privetb | Ligustrum spp. | SW, SH, HW |
| Pyracantha; Firethorn | Pyracantha spp. | SH |
| Rhododendron | Rhododendron spp. | SH, HW |
| Spruce | Picea spp. | SW, HW |
| Viburnum | Viburnum spp. | SW, HW |
| Yew | Taxus spp. | SH, HW |
| a SW = softwood, SH = semi-hardwood, HW = hardwood b Plants considered moderately weedy in the eastern United States. Unless working with either a sterile or demonstrated functionally sterile cultivar, use care when distributing.
|
||
Procedures for Rooting Woody Stem Cuttings
Cuttings should generally consist of the current or past season's growth. Avoid material with flower buds. Remove any flowers and flower buds when preparing cuttings so the cuttings' energy can be used in producing new roots rather than flowers. Take cuttings from healthy, disease-free plants.
The fertility status of the stock (parent) plant can influence rooting. Avoid taking cuttings from plants that show symptoms of nutrient deficiency. Conversely, plants that have been fertilized heavily, particularly with nitrogen, also may not root well. The stock plant should not be under water stress. In general, cuttings taken from young plants root quicker than cuttings taken from older, more mature plants. Cuttings from lateral shoots often root better than cuttings from terminal shoots.
Early morning is the best time to take cuttings because the plant is fully turgid. It is important to keep the cuttings cool and moist until they are stuck. An ice chest or a dark plastic bag with wet paper towels may be used to store cuttings. If there will be a delay in sticking cuttings, store them in a plastic bag in a refrigerator.
While terminal bud ends of the stem are best, a long shoot can be divided into several cuttings. Cuttings are generally 4 to 6 inches long. Use a sharp, thinbladed pocketknife or sharp pruning shears. Dip the cutting tool in rubbing alcohol or a mixture of one part bleach to nine parts water to prevent transmitting diseases from infected plant parts to healthy ones.
Remove the leaves on the lower one-third to one-half of the cutting (Figure 13–11). On large-leafed plants, the remaining leaves may be cut in half perpendicular to the midvein to reduce moisture loss and conserve space in the rooting area.
| Common Name | Scientific Name | Type of Cuttinga |
|---|---|---|
| Basswood, American, American linden | Tilia americana | SW, SH |
| Azalea (deciduous) | Rhododendron spp. | SW |
| Beautyberry | Callicarpa bondinieri | SW |
| Birch | Betula spp. | SW |
| Bittersweetc | Celastrus spp. | SW, SH, HW |
| Blueberry | Vaccinium spp. | SW, HW |
| Broom, Scotch, Scottish | Cytisus scoparius | SW, HW |
| Catalpa | Catalpa spp. | SW, HW |
| Cherry, Flowering | Prunus spp. | SW, SH |
| Clematis | Clematis spp. | SW, SH |
| Crabapple | Malus spp. | SW, SH |
| Crapemyrtle, Crape Myrtle | Lagerstroemia indica | SW, HW |
| Deutzia | Deutzia spp. | SW, HW |
| Dogwood | Cornus spp. | SW, SH |
| Elderberry | Sambucus spp. | SW |
| Elm | Ulmus spp. | SW |
| Euonymus | Euonymus spp. | SH, HW |
| Forsythia | Forsythia spp. | SW, SH, HW |
| Fringe tree; Fringetree | Chionanthus spp. | SW |
| Ginkgo; Maidenhair tree | Ginkgo biloba | SW |
| Goldenrain tree | Koelreuteria spp. | SW |
| Hibiscus, Chinese | Hibiscus rosa-sinensis | SW, SH, HW |
| Honeylocust; Honey locust | Gleditsia triacanthos | HW |
| Honeysucklec | Lonicera spp. | SW, SH, HW |
| Hydrangea | Hydrangea spp. | SW, SH, HW |
| Ivy, Boston | Parthenocissus tricuspidata | SW, HW |
| Larch | Larix kaempferi | SW, HW |
| Lilac | Syringa spp. | SW |
| Maple | Acer spp. | SW, SH |
| Mock orange | Philadelphus inodorus | SW, HW |
| Mulberry | Morus spp. | SW, SH, HW |
| Pear, Calleryb | Pyrus calleryana | SH |
| Poplar, Aspen, Cottonwood | Populus deltoides | SW, HW |
| Poplar, Yellow; Tulip poplar | Liriodendron tulipifera | SH |
| Quince, Flowering | Chaenomelea spp. | SW, SH |
| Redbud | Cercis spp. | SW, SH |
| Redwood, Dawn | Metasequoia glyptostroboides | SW, HW |
| Rose | Rosa spp. | SW, SH, HW |
| Rose-of-Sharon, Shrub-althea | Hibiscus syriacus | SW, HW |
| Russian olive, Oleaster | Elaeagnus angustifolia | SH |
| Serviceberry | Amelanchier spp. | SW |
| Smoketree; Smokebush | Cotinus coggygria | SW |
| Spirea | Spiraea spp. | SW |
| St. John's Wort | Hypericum spp. | SW |
| Sumac | Rhus spp. | SW |
| Sweetgum | Liquidambar styraciflua | SW |
| Trumpet creeper | Campsis spp. | SW, SH, HW |
| Virginia creeper | Parthenocissus quinquefolia | SW, HW |
| Weigela | Weigela florida | SW, HW |
| Willow | Salix spp. | SW, SH, HW |
| Wisteriab | Wisteria spp. | SH, HW |
| a SW = softwood, SH = semi-hardwood, HW = hardwood b Plants considered moderately weedy. Unless working with a sterile variety use care when distributing.
|
||
During preparation of stem cuttings of particular woody species, the lower portion of the stem that is inserted into the rooting media is deliberately wounded, which is done to stimulate rooting. Most species do not require wounding, but some benefit greatly from this practice.
There are two general types of wounds, light and heavy. A light wound consists of two or four equidistant vertical cuts, administered with a knife or single-edge razor blade, to the lower portion (approximately 1 to 1½-inches) of the cutting. The cuts go through the bark into the wood but are not deep enough to split the stem. Many conifer cuttings respond to light wounds. On the other hand, a heavy wound consists of removal of a thin strip of bark on one or opposite sides of the lower 1 to 1½-inches of a cutting, exposing the green tissue just beneath the bark termed the cambium. Be careful when applying a heavy wound not to remove (scrape) all the bark from the lower portion of the stem as this kills the cutting or prevents it from rooting. Species that respond to heavy wounding include magnolias and evergreen rhododendrons. As mentioned previously, most species do not require wounding. There is nothing wrong, however, with using wounding, whether it be light or heavy, on all species provided it is done properly. Also, wounding by itself is of no benefit unless cuttings are treated with root-promoting compounds after wounding.
Treating cuttings with root-promoting compounds (chemicals), particularly for difficult-to-root species, is a common practice when rooting woody stem cuttings because these compounds increase the percentage of cuttings that form roots, speed up the rooting process (make the cuttings root faster), increase the uniformity of rooting, and lastly increase the number and quality of roots produced per cutting. Root-promoting compounds are generally available commercially in two forms, powders or liquids.
When treating cuttings with root-promoting compounds, prevent possible contamination of the entire supply (stock) of formulation by putting some in a separate container before treating cuttings. Any material remaining after treatment should be discarded and not returned to the original container. Be sure to tap the lower portion of the cutting to remove excess material when using a powder formulation. If the base of the cutting is not moist, moisten it with water before applying a powder formulation so the powder adheres to the base. Once the cuttings have been treated with a powder formulation, use a dibble for inserting them into the rooting medium so the powder stays on during insertion. When treating cuttings with a liquid formulation of a root-promoting compound, dip the lower ½-inch to 2 inches of a cutting into the solution for 1 to 2 seconds followed by 15 to 20 minutes of air drying; then insert the cutting into the rooting medium without the use of a dibble.
Use a rooting medium such as coarse sand, pine bark, or a mixture of one part peat and one or two parts perlite (by volume), or peat and sand (1:1 by volume). The rooting medium should be sterile, low in fertility, sufficiently well drained to permit aeration, and moisture retentive enough so that it does not have to be watered too frequently. Moisten the medium before inserting cuttings.
Various commercial potting mixes can be used. Insert the cuttings one-third to one-half of their length into the medium with the buds pointing upward. Do not insert the stem upside down. Space cuttings just far enough apart to allow all leaves to receive sunlight. Water again after inserting the cuttings if the containers or frames are 3 or more inches deep. Cover the cuttings with plastic and place in indirect light. Keep the medium moist until the cuttings have rooted. Rooting is improved if the plants are misted on a regular basis.
Rooting time varies with the type of cutting, the species being rooted, and environmental conditions. Conifers require more time than broadleaf plants. Late fall or early winter is a good time to root conifers. Once rooted, conifer cuttings may be left in the rooting structure until spring.
Root Cuttings
Some plants can be propagated from a section of a root. Root cuttings are usually taken from 2- to 3-year-old plants during the dormant season when carbohydrate levels are high. Root cuttings of some species produce new shoots, which then form their own root system, whereas root cuttings of other plants develop root systems before producing new shoots. Examples of plants that can be propagated from root cuttings include blackberry, apple, fig, lilac, phlox, raspberry, rose, sumac, and trumpet vine.
Plants with large diameter roots are normally propagated outdoors. The root cuttings should be 2 to 6 inches long. Make a straight cut on the proximal end (nearest the crown of the parent plant) and a slanted cut on the distal end (farthest from the crown) of each root cutting. Tie the cuttings in bundles with all the same ends together. It is important to maintain the correct polarity of the cuttings. Store about three weeks in moist sawdust, peat moss, or sand at 40°F. Remove from storage. Plant the cuttings distal end pointed down, about 2 to 3 inches apart in well-prepared garden soil. The tops of the cuttings (proximal ends) should be the shallowest part of the planting at 2 to 3 inches below the soil surface.
For plants with small diameter roots, cut the roots into 1-inch to 2-inch sections. Lay the cuttings horizontally on the medium surface in a flat and cover with about ½-inch of soil or sand. Place the flat inside a plastic bag or cover with a pane of glass. Place the flat in the shade and remove the protective cover after new shoots appear.

Figure 13–6. Sanseviera trifasciata (snake plant) leaves that were cut and placed in rooting medium.
Chris Alberti CC BY 2.0
Asexual Propagation - Other Methods
LAYERING
Stems still attached to their parent plant may form roots where they come in contact with a rooting medium. This method of vegetative propagation is generally successful because water stress is minimized and carbohydrate and mineral nutrient levels are high. The development of roots on a stem while the stem is still attached to the parent plant is called layering. A layer is a rooted stem after it has been removed from the parent plant. Some plants propagate naturally by layering, but sometimes plant propagators assist the process. Layering is enhanced by wounding the side of the stem where the roots will grow or by bending the stem very sharply. The rooting medium should always provide aeration and a constant supply of moisture. Some common forms of layering are as follows. However, keep in mind these protocols are sometimes modified.
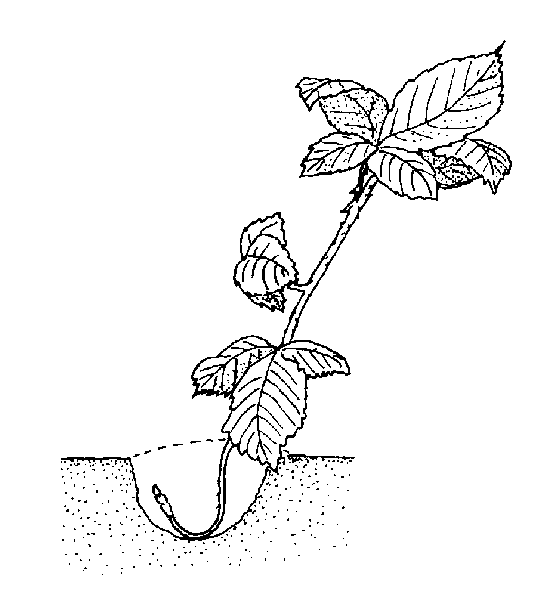
Tip layering. Dig a hole 3 to 4 inches deep in the rooting medium. Insert the tip of a current season's shoot and cover it with soil. The tip grows downward first, then bends sharply and grows upward (Figure 13–12). Roots form at the bend. The recurved tip becomes a new plant. Remove the tip layer and plant it in late fall or early spring. Examples of plants propagated this way include purple and black raspberries, trailing blackberries, and dewberries. The aforementioned plants also do this naturally.
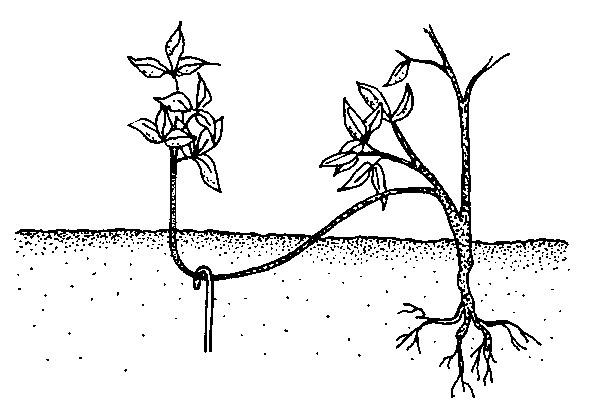
Simple layering. Bend the stem to the ground. Cover part of it with soil, leaving the remaining 6 to 12 inches above the soil. Bend the tip into a vertical position and stake in place (Figure 13–13). The sharp bend often induces rooting, but wounding the lower side of the bent branch may help. Simple layering can be done on most plants that have low-growing branches. Examples include azalea, forsythia, boxwood, honeysuckle, rhododendron, and wax myrtle.
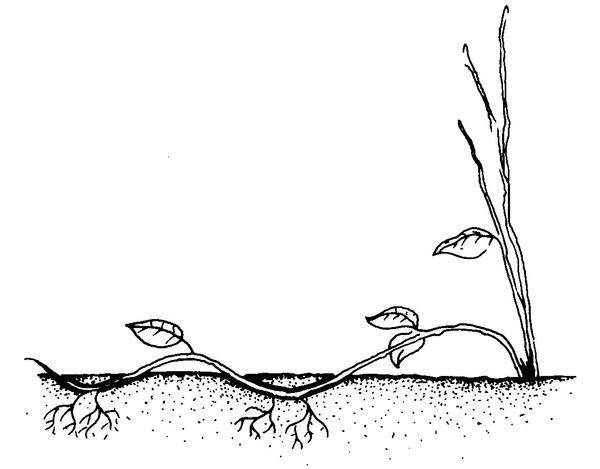
Compound (serpentine) layering. Bend the stem to the rooting medium as for simple layering, but alternately cover and expose stem sections. Wound the lower side of the stem sections to be covered (Figure 13–14). This method works well for plants producing vinelike growth such as heart-leaf philodendron, pothos, and grape.
Mound (stool) layering. Cut the plant back to 1 inch above the ground in the dormant season. Dormant buds produce new shoots in the spring. Mound soil over the new shoots as they grow (Figure 13–15). Roots develop at the bases of the young shoots. Remove the layers in the dormant season. Mound layering works well on apple rootstocks, cotoneaster, daphne, quince, and spirea.
Air layering (pot layering, circumposition, marcottage, Chinese layering, gootee). Air layering can be used to propagate large, overgrown houseplants (such as rubber plants or dieffenbachia that have lost most of their lower leaves) as well as some woody ornamentals such as camellias. The process varies depending on whether the plant is a monocot or dicot. For monocots, such as Dracaena fragrans ‘Massangeana’ (corn plant), make an upward cut about one-third of the way through the stem. This is normally done on a stem about 1 foot from the tip. The cut is held open with a toothpick or wooden matchstick. Dust the wound with rooting hormone and surround with damp, unmilled sphagnum moss. Wrap the moss with plastic and hold in place with twist ties or electrician's tape. Aluminum foil can also be used; it does not require twist ties or tape to hold it in place.
The process for dicots is similar except a 1-inch ring of bark is removed from the stem. Scrape the newly bared ring to remove the cambial tissue in order to prevent callus from forming. Wrap and cover using the same procedure as that described for monocots (Figure 13–16).
After the rooting medium is filled with roots, sever the stem below the medium. The new plant requires some pampering after planting until the root system becomes more developed.
Natural forms of layering. Sometimes layering happens without the help of a propagator. Runners and offsets are specialized structures that facilitate propagation by layering.
A runner produces new shoots where it touches the growing medium. Plants that produce stolons or runners are propagated by severing the new plants from their parent stems. Plantlets at the tips of runners may be rooted while still attached to the parent or detached and placed in a rooting medium. Examples include strawberry and spider plants (Figure 13–17).
Plants with a rosetted stem often reproduce by forming new shoots, called offsets (offshoots), at their base or in the leaf axil. Sever the new shoots from the parent plant after they have developed their own root system. Unrooted offsets of some species may be removed and placed in a rooting medium. Some of these must be cut off, whereas others may simply be lifted from the parent stem. Examples include date palm, haworthia, bromeliads, and many cacti.
SEPARATION AND DIVISION
Separation and division are the easiest and quickest ways to propagate many plants. Separation uses naturally occurring vegetative structures such as bulbs and corms. Individual bulbs or corms are separated from a clump.
Most of the spring- and summerflowering bulbs are propagated this way. Division involves digging up the plant or removing it from its container and cutting (dividing) the plant into separate pieces. Division uses specialized vegetative structures such as rhizomes and tubers. Indoor plants that can be propagated by division include ferns, snake plant, prayer plant, and African violet. Outdoor plants that can be divided include many perennials such as daylily, hosta, iris, liriope, and verbena. Separation and division are normally done in the fall or early spring.
BUDDING AND GRAFTING
Budding and grafting are methods of asexual propagation that join parts of two or more different plants together so they unite and grow as one plant. These techniques are used to propagate cultivars that do not root well from cuttings or to alter some aspect of the plant (for example, to create weeping or dwarf forms). Most fruit and nut trees are propagated by budding or grafting.
The scion consists of a short stem piece with one or more buds and is the part of the graft that develops into the top of the grafted plant. The rootstock (also called stock or understock) provides the new plant's root system and sometimes the lower part of the stem. Seedling rootstocks are used commonly.
Six conditions must be met for bud grafting to be successful:
- The scion and rootstock must be compatible (capable of uniting).
- The scion and stock plant must be at the proper physiological stage of growth.
- The cambial region of the scion and the stock must be in close contact—touching if possible.
- Proper polarity must be maintained (the buds on the scion must be pointed upward).
- Immediately after grafting, all cut surfaces must be protected from drying out.
- Proper care must be given to the graft after grafting. (See "Care of Buds and Grafts").
Once the bud has healed and broken to form a shoot, the top of the stock plant is cut back (removed) to the bud union.
Not all plants can be grafted. The two plants should be closely related taxonomically. Grafting between clones and seedlings of the same species is usually successful.
Budding
Budding, or bud grafting, is the union of one bud, with or without a small piece of bark, from one plant (scion) onto a stem of a rootstock. It is especially useful when scion material is limited. Budding is usually done during the growing season when the bark is slipping (soft and easy to peel back from the cambium), June through August in the Southeast United States, but it can also be done in late winter or early spring.
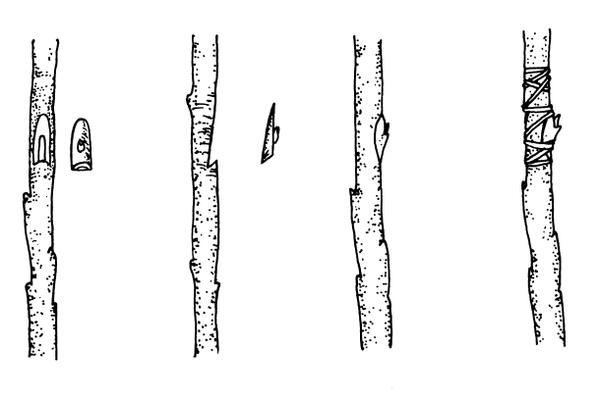
T-budding—This is the most commonly used budding technique (Figure 13–18). When the bark is slipping, make a vertical cut (same axis as the rootstock) through the bark of the rootstock, avoiding any buds on the stock. Make an intersecting, horizontal cut at the top of the vertical cut (forming a “T” shape), and loosen the bark by twisting the knife slightly at the intersection. Remove a shield-shaped piece of the scion, including a bud, some bark, and a thin section of wood. Push the shield under the loosened stock bark at the "T." Wrap the union, leaving the bud exposed. Many fruit trees are propagated by this method.
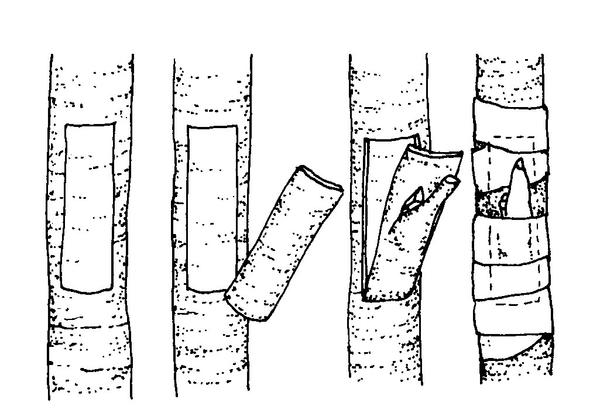
Chip budding—This method can be used when the bark is not slipping. Although all the basics in handling budwood and stock are the same for chip budding and T-budding, the cuts made in chip budding differ radically. The first cut on both stock and scion is made at a 45o to 60o downward angle to a depth of about ⅛-inch (Figure 13–19). After making this cut on a smooth part of the rootstock, start the second cut about ¾-inch higher and draw the knife down to meet the first cut. (The exact spacing between the cuts varies with species and the size of the buds.) Then remove the chip. Cuts on both the scion (to remove the bud) and the rootstock (to insert the bud) should be exactly the same size. Although the exact location is not essential, the bud is usually positioned one-third of the way down from the beginning of the cut. If the bud shield is significantly narrower than the rootstock cut, line up one side exactly. Wrapping is extremely important in chip budding. If all exposed edges of the cut are not covered, the bud dries out before it can “take.” Chip budding has become more popular over the past five years because of the availability of thin (2 mil) polyethylene tape as a wrapping material. This tape is wrapped to overlap all of the injury, including the bud (Figure 13–20), and forms a miniature plastic greenhouse over the healing graft.
Grafting
Grafting is the union of the stems of two plants to grow as one. There are several kinds of grafting; which method to use depends on the age and type of plants involved.
Whip and tongue grafting—This method is often used for stems ¼-inch to ½-inch in diameter. The scion and rootstock are usually of the same diameter. This graft provides excellent cambial contact and heals quickly. Make a sloping cut 1-inch to 2½-inches long at the top of the rootstock and a matching cut on the bottom of the scion. On the cut surface, slice downward into the stock and up into the scion, so the pieces will interlock when brought together. Fit the pieces together such that the cambium matches on at least one side, and then wrap and tie or wax the graft union (Figure 13–21).
Cleft grafting—Cleft grafting is one of the oldest and most widely used methods of grafting. The two most common circumstances when one would use this technique are (1) when creating a weeping ornamental tree and (2) when upgrading an older cultivar of a fruit tree to a newer, improved one while maintaining the existing root system. Cleft grafting is best done in early spring when the buds on the stock plant are beginning to swell but before active growth has begun. The scions, however, must be fully dormant. Collect scion wood ⅜ to ⅝ inch in diameter during the dormant season and refrigerate it until needed.
Cut the limbs to be reworked at a right angle to the main axis of the branch. Use a heavy knife to split the branch, making a 2-inch vertical cut through the center of the stub to be grafted. Be careful not to tear the bark. Keep this cut wedged apart. Prepare two scion pieces 3 to 5 inches long; cut the lower end of each scion piece into a wedge. Insert the scions at the outer edges of the cut in the stock. Tilt the top of the scion slightly outward and the bottom slightly inward to be sure the cambial layers of the scion and stock touch. Remove the wedge propping the slit open, and cover all cut surfaces with grafting wax (Figure 13–22). If both scions grow, one can be removed later.
Some other types of grafting include splice, side, stub, side veneer, and side tongue. Check reference books for additional information. Each method has advantages for special situations.
Care of Buds and Grafts
Propagation is not likely to be successful unless you properly care for the plants for a year or two after budding or grafting. If you use binding materials such as waxed string or nursery tape, remove them shortly after growth starts to prevent girdling. Rubber budding strips have some advantages over other materials. They expand with growth and usually do not need to be removed because they deteriorate after several months. Inspect the grafts after two or three weeks to see if the grafting wax has cracked and, if necessary, rewax the exposed surface. After this, the union will probably have healed and no more waxing will be necessary.
Limbs on the old cultivar (rootstock) that are not selected should be cut back at the time of grafting. The total leaf surface of the old cultivar should be reduced gradually as the new one increases for one or two years; by this time, the new cultivar (scion) has had adequate time to grow. Completely removing all the limbs of the old cultivar at the time of grafting increases the shock to the tree and causes excessive suckering. Also, the scions may grow too fast, making them susceptible to wind damage.
MICROPROPAGATION
Micropropagation involves the application of tissue culture techniques to propagate plants from very small plant parts (parts of leaves, stems, shoot tips, root tips, single cells, and pollen grains). The small plant part is grown (cultured) in a test tube, petri dish, or other sterilized container with a culture medium and precise environmental conditions. The container and growing medium must be sterilized. Because plants often harbor bacterial and fungal spores, the plant tissue must also be disinfected.
Micropropagation is a rapidly growing part of the plant propagation industry. It is not practical for most home gardeners because of the very specific requirements of the culture media and the constant efforts that must be made to avoid possible contamination from disease organisms. For nurseries, special care must be taken in transporting micropropagated plants from the lab to the store because they are not acclimated to outdoor growing conditions.

Figure 13–16. Air layering. Remove a 1-inch strip of bark around the stem, dust rooting hormone on the cut surface, cover with moist sphagnum moss, enclose in plastic or aluminum foil. Rooting hormone is not always necessary.
Velacreations, Flickr CC BY 2.0

Figure 13–17. Some plants, such as the spider plant, produce runners that are propagated easily.
Christ Alberti CC BY 2.0
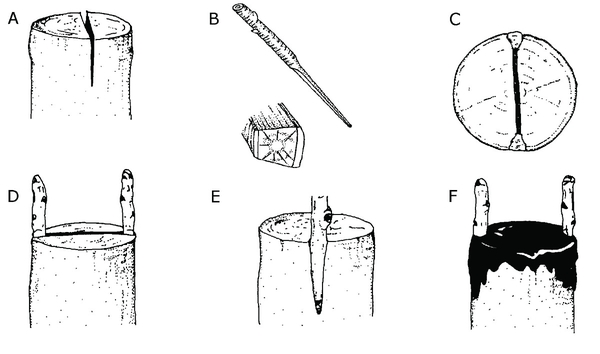
Figure 13–22. Cleft grafting. A) Split the branch of the rootstock to be grafted with a 2-inch vertical cut. B) Prepare two scion pieces 3 to 5 inches long with wedge-shaped ends. C) and D) Insert the scions into the outer edges of the stock. E) Make sure the cambial layers of the scion and stock touch. F) Cover all cut surfaces with grafting wax.
Hardening Plants
Hardening is the process of conditioning a plant for growth outdoors. If plants produced inside are planted outdoors without a hardening period, their growth could be severely limited. Hardening is most critical with early crops, when adverse climatic conditions can be expected.
Hardening is accomplished by decreasing temperature and relative humidity gradually, and reducing water. This procedure results in the accumulation of carbohydrates and thickening of cell walls. A change from a soft, succulent type of growth to a firmer, harder type is desired.
The process should be started at least two weeks before plants are to be planted in the garden. Place seedlings outside in a protected area on warm days (Figure 13–23). Increase the length of exposure gradually. Do not put tender seedlings outdoors on windy days or when temperatures are below 45°F. Even cold-hardy plants will be injured if exposed to freezing temperatures before they are hardened.
The hardening process is intended to slow plant growth. Carried to an extreme, however, hardening can cause significant damage. For example, cauliflower produces thumb-sized heads and fails to develop further if hardened too severely; cucumbers and melons stop growing entirely.
Genetically Modified Organisms (GMOs)
Genetically modified organisms (GMOs) have their DNA manipulated through the introduction of genes from other organisms or through the rearrangement of their own genes. These changes are made to produce an organism with a desired trait that is not naturally occurring. Genetic modifications to plants can include resistance to insect pests or diseases, the ability to grow faster, the ability to use less water, or increased resistance to pesticides or herbicides. Three federal agencies currently regulate GMOs: the U.S. Department of Agriculture (USDA), the Food and Drug Administration (FDA), and the Environmental Protection Agency (EPA). Much debate exists over the sustainability and environmental impact of these genetic modifications, and the jury is still out. Currently, in the United States, food and seeds are not required to be labeled if they contain GMOs. The only label that guarantees no GMOs is the “USDA Certified Organic” label (Figure 13–24).
Frequently Asked Questions
1. When should I collect oak acorns and other shade tree seeds to grow them, and how should I handle the seeds to get them to germinate?
Seeds that mature and drop from trees in the fall should be collected in the fall and sown immediately in tilled and prepared beds or into containers. They generally germinate the following spring. Before planting, place acorns in water. Sound acorns sink; unviable acorns float. Discard the floaters. The cap should also be removed from the acorn. Dogwood and magnolia seed should have the outer red covering removed; these seeds can be soaked to ferment the pulpy flesh from the seed. Red maple seeds ripen in spring and should be collected and sown immediately after removing the dry samaras in prepared beds or into pots. Check references on handling woody species from seeds. The Manual of Woody Landscape Plants: Their Identification, Ornamental Characteristics, Culture, Propagation and Uses by Michael A. Dirr is a highly recommended source of information. Another excellent source for the propagation of woody species is The Reference Manual of Woody Plant Propagation: From Seed to Tissue Culture, by Michael A. Dirr and Charles W. Heuser Jr. For showy native woody plants, consult Growing and Propagating Showy Native Woody Plants by Richard E. “Dick” Bir (available from The University of North Carolina Press; or call 800-848-6224 to order).
2. My jade plant is dying. Can I propagate it and keep it alive?
Cuttings from healthy plants work best; sometimes, cuttings from plants that are struggling will not take root so it may not be possible to propagate it. If you would like to try, jade plants are propagated by stem or leaf cuttings. If it is a variegated jade, use a stem cutting to ensure you are preserving the genetic material that leads to the variation in leaf color.
3. I planted seeds for some annual flowers, but none of them came up. Why?
There could be several reasons your seeds failed to germinate.
- The seeds were never viable.
- They were planted too deep or too shallow.
- The seedbed was kept too wet or too dry.
- They died shortly after germinating.
- The seeds or seedlings were eaten by an insect or animal.
- The seeds or seedlings were killed by a disease.
- A weather event such as frost killed the seedlings.
4. How do I propagate azaleas?
One of the most reliable ways to propagate azaleas is to layer some of the lower branches in the soil and weigh them down with a brick (wounding and applying a rooting hormone also helps the process). If cuttings are to be used, best success is achieved on evergreen azaleas with semi-hardwood cuttings (they break with a snap), taken late June through August. Again, wounding the lower stems and applying a rooting hormone increases success rates. Be sure to use a well-drained medium and provide partial shade. Roots should form in four to six weeks. These Horticulture Information Leaflets from NC State Extension are excellent references: HIL 8701, Plant Propagation by Layering: Instructions For the Home Gardener, and HIL 8702, Plant Propagation by Stem Cuttings: Instructions For the Home Gardener.
5. My irises are about 10 years old, and they are not blooming well any more. What is wrong with them?
Irises produce leaves and flowers from thick rhizomes beneath the ground. In time the rhizome produces side shoots and the original rhizome withers and dies. The smaller side shoots need to be broken off and planted separately so they can mature into a new plant. Irises should be divided every three to four years for the plants to remain healthy and vigorous. Read more about irises and their propagation NC State Extension publication HIL-8506, Bearded Iris for the Home Landscape.
*To read other chapters in the North Carolina Extension Gardener Handbook see the Table of Contents. If you have questions about this chapter contact your local NC State Extension expert at your local Cooperative Extension Center.
Further Reading
Beyl, Caula A. and Robert N. Trigiano, eds. Plant Propagation: Concepts and Laboratory Exercises. Boca Raton, Florida: CRC Press, 2008. Print.
Bir, Richard E. Growing and Propagating Showy Native Woody Plants. Chapel Hill, North Carolina: The University Of North Carolina Press, 1992. Print.
Bonner, Franklin T. and Robert P. Karrfalt, eds. The Woody Plant Seed Manual. Washington, DC: United States Department of Agriculture Forest Service, 2008. Print and PDF file. Agriculture Handbook 727.
Bryant, Geoff. Propagation Handbook: Basic Techniques for Gardeners. Mechanicsburg, Pennsylvania: Stackpole Books, 1995. Print.
Dirr, Michael A. Manual of Woody Landscape Plants: Their Identification, Ornamental Characteristics, Culture, Propogation and Uses. 6th ed. Champaign, Illinois: Stipes Publishing L.L.C., 2009. Print.
Dirr, Michael A., and Charles W. Heuser, Jr.. The Reference Manual of Woody Plant Propagation: From Seed to Tissue Culture. 2nd ed. Cary, North Carolina: Varsity Press, Inc., 2006. Print.
Hartmann, Hudson T., et al.. Hartmann & Kester's Plant Propagation: Principles and Practices. 8th ed. Upper Saddle River, New Jersey: Prentice Hall, Inc., 2010. Print.
Hill, Lewis. Secrets of Plant Propagation: Starting Your Own Flowers, Vegetables, Fruits, Berries, Shrubs, Trees, and Houseplants. North Adams, Massachusetts: Story Publishing, 1985. Print.
Phillips, Harry R. Growing and Propagating Wild Flowers. Chapel Hill, North Carolina: The University of North Carolina Press, 1985. Print.
Royal Horticultural Society Propagation Techniques. London: Octopus Publishing Group Ltd, 2013. Print.
Thompson, Peter. Creative Propagation. 2nd ed. Portland, Oregon: Timber Press, Inc., 2005. Print.
Toogood, Alan, ed. American Horticultural Society Plant Propagation: The Fully Illustrated Plant-by-Plant Manual of Practical Techniques. New York: DK Publishing, Inc., 1999. Print.
Chapter Text Hyperlinks
Low Investment Propagation/Winter Protection Structure, HIL-404
A Simple Intermittent Mist System For Propagation, HIL-405
A Small Backyard Greenhouse for the Home Gardener, AG-426
Growing and Propagating Showy Native Woody Plants, by Richard E. Bir
Plant Propagation by Layering, HIL-8701
Plant Propagation by Stem Cuttings, HIL-8702
Bearded Iris for the Home Landscape, HIL-8506
For More Information
NC State Resources
- Bearded Iris for the Home Landscape, HIL-8506
- Grafting and Budding Nursery Crop Plants, AG-396
- How to Propagate a Butterfly Bush
- Low Investment Propagation/Winter Protection Structure, HIL-404
- Nursery Crop Science Commercial Horticulture Information Portal
- Overcoming Seed Dormancy: Trees and Shrubs, HIL-8704
- Plant Propagation by Layering, HIL-8701
- Plant Propagation by Leaf, Cane and Root Cuttings, HIL-8700
- Plant Propagation by Stem Cuttings, HIL-8702
- Propagation by Cutting
- A Small Backyard Greenhouse for the Home Gardener, AG-426
More NC State Resources
Other Resources
- Growing and Propagating Showy Native Woody Plants, by Richard E. Bir
Contributors
Authors:
Anthony V. LeBude, Associate Professor, Department of Horticultural Science
Frank A. Blazich, Distinguished Professor Emeritus, Department of Horticultural Science
Contribution by Extension Agents: Jessica Strickland, Mary Hollingsworth, Shawn Banks, Mack Johnson, Paige Patterson, Peg Godwin
Contributions by Extension Master Gardener Volunteers: Patty Brown, Renee Lampila, Jackie Weedon, Karen Damari, Connie Schultz
Content Editors:
Lucy Bradley, Associate Professor and Extension Specialist, Urban Horticulture, NC State University; Director, NC State Extension Master Gardener program;
Kathleen Moore, Urban Horticulturist
Copy Editor: Barbara Scott
Based on text from the 1998 Extension Master Gardener manual prepared by:
Erv Evans, Extension Associate, Department of Horticulture Science
Frank A. Blazich, Professor, Department of Horticulture Science
How to cite this chapter:
LeBude, A.V., and F.A. Blazich. 2022. Propagation, Chapter 13. In: K.A. Moore, and. L.K. Bradley (eds). North Carolina Extension Gardener Handbook, 2nd ed. NC State Extension, Raleigh, NC. <http://content.ces.ncsu.edu/13-propagation>
Publication date: Feb. 1, 2022
AG-831
Other Publications in North Carolina Extension Gardener Handbook
- 1. Soils & Plant Nutrients
- 2. Composting
- 3. Botany
- 4. Insects
- 5. Diseases and Disorders
- 6. Weeds
- 7. Diagnostics
- 8. Integrated Pest Management (IPM)
- 9. Lawns
- 10. Herbaceous Ornamentals
- 11. Woody Ornamentals
- 12. Native Plants
- 13. Propagation
- 14. Small Fruits
- 15. Tree Fruit and Nuts
- 16. Vegetable Gardening
- 17. Organic Gardening
- 18. Plants Grown in Containers
- 19. Landscape Design
- 20. Wildlife
- 21. Youth, Community, and Therapeutic Gardening
- Appendix A. Garden Journaling
- Appendix B. Pesticides and Pesticide Safety
- Appendix C. Diagnostic Tables
- Appendix D. Garden Tools
- Appendix E. Season Extenders and Greenhouses
- Appendix F. History of Landscape Design
- Appendix G. Permaculture Design
- Appendix H. Community Gardening Resources
- Appendix I. More NC State Resources
- Glossary
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.