The pests of beans, southern peas, and English peas are a diverse group. Mites and beetles are usually the most common pests of beans. Aphids frequently infest English peas, and stink bugs and leaffooted bugs are nuisances on southern peas. Some aphids transmit viruses.
- Pests that feed primarily on foliage
- Insects that mine or eat holes in foliage
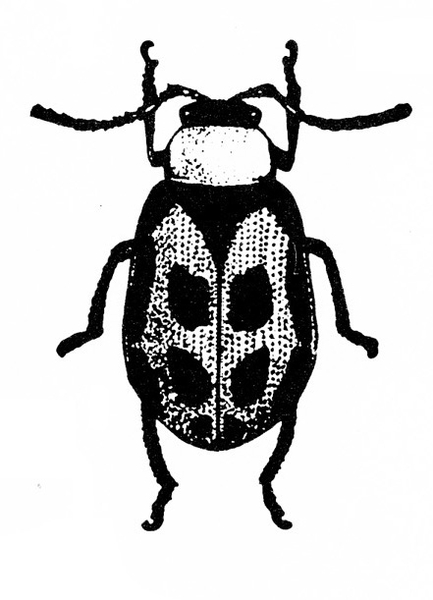
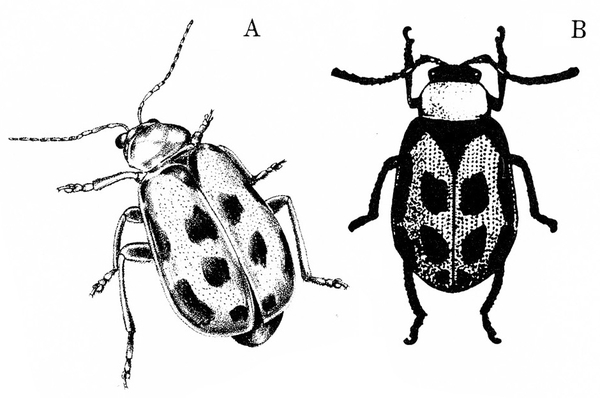
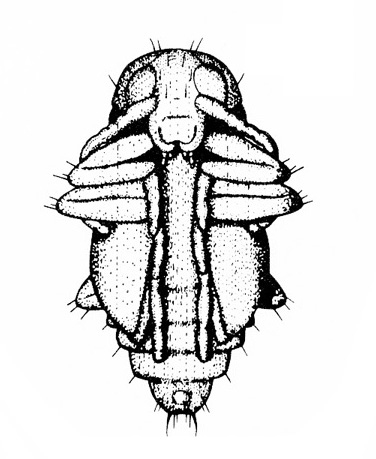
- Bean leaf beetle —These reddish to yellowish-brown beetles are 3/16 to 1/4 inch long and often have three black spots on each wing cover (Figure 1). They have black margins and a black triangle on the front portion of the wings. Bean leaf beetles consume mostly young leaves, although they sometimes attack the outer wall of pods when vegetative growth ends.
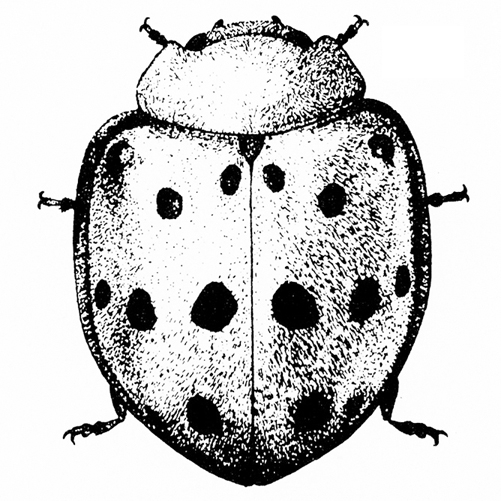
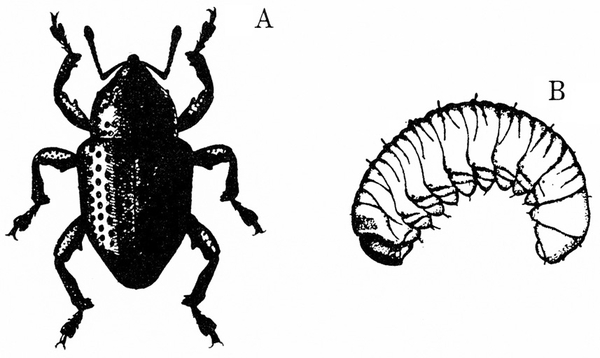
- Mexican bean beetle—Copper-red, dome-shaped beetles are 1/4 to 5/16 inch long; each wing cover has eight small, black spots that form three rows across the body when wings are at rest (Figure 2). These beetles skeletonize leaves, which become lacelike and turn brown from this feeding.
- Vegetable leafminer—Colorless to bright yellow, these maggots grow as long as 1/8 inch (Figure 3). The head is pointed. The maggots make serpentine mines that get wider as the maggot grows. (For more information about vegetable leafminers, see "Pests of Tomato.")
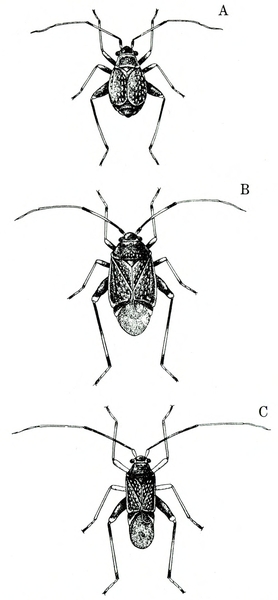
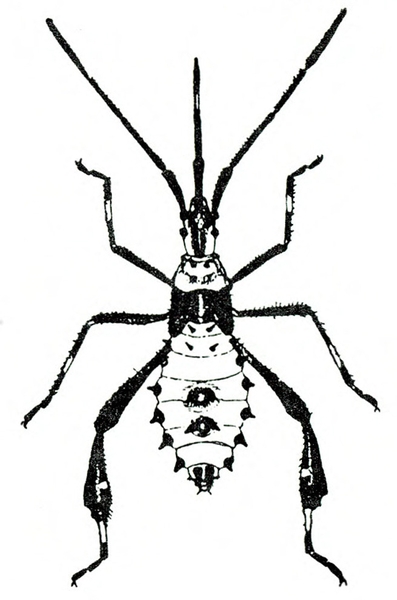
- Garden fleahopper—These small, black bugs are about 1/32 inch to a little more than 1/16 inch long and have three, distinct forms (Figure 4A–C). Nymphs are smaller and yellow to dark green, with a black spot on each side. They suck sap from foliage. Severe infestation can cause major defoliation, reducing crop yield. All stages of garden fleahoppers jump readily. (For more information about garden fleahoppers, see "Pests of Cucurbits.")
- Sap-sucking pests that cause discoloration, deformation, or abscission (detachment of plant parts)
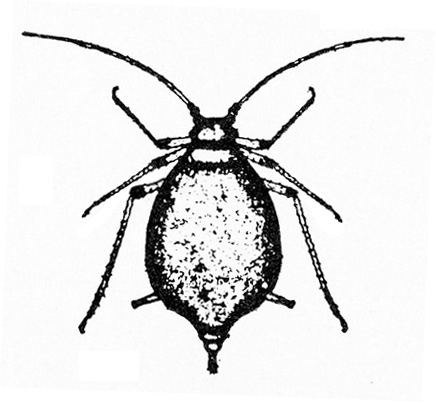
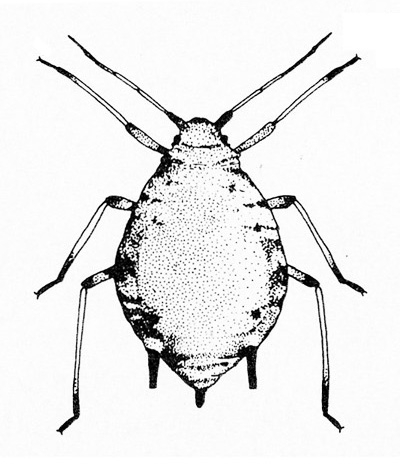
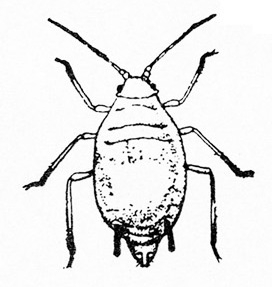
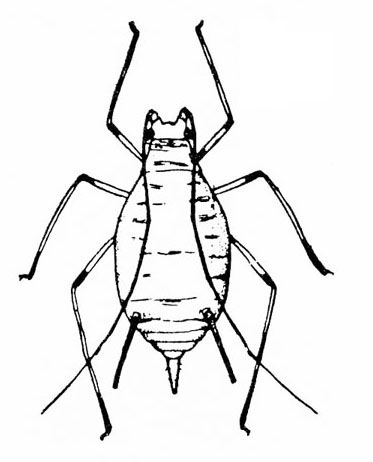
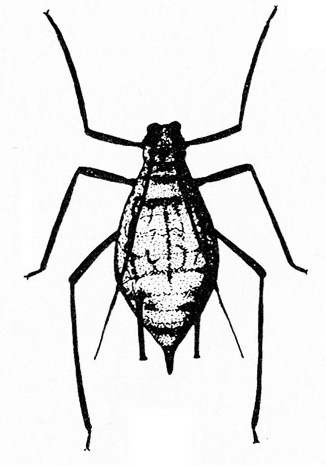
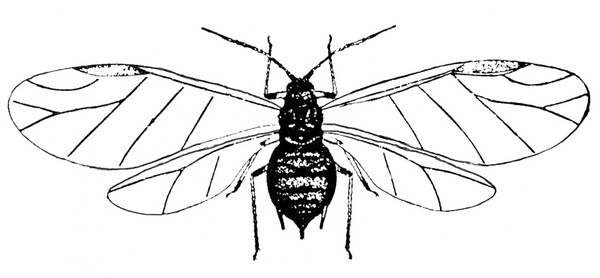
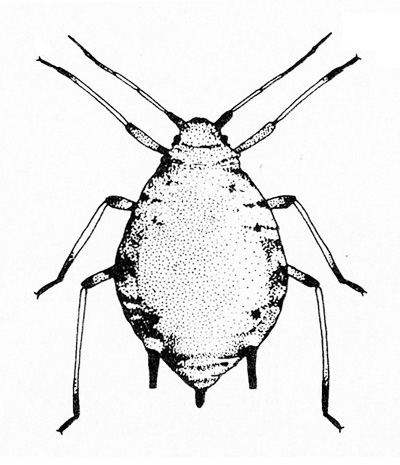
- Aphids—Aphids are soft-bodied, pear-shaped insects with a pair of dark cornicles (tailpipe-like appendages) and a cauda (tail) protruding from the abdomen. They may be winged or wingless, with wingless forms most common. Aphids feed in colonies and cause discoloration, curling, and deformation of foliage. They often transmit viruses, and they excrete honeydew on which sooty mold grows.
- Bean aphid —Dark-green to black, bean aphids have white appendages. These aphids grow to almost 1/8 inch long, and the cornicles are about the same length as the cauda (Figure 5A). Nymphs are green. Mature nymphs have five to seven pairs of white spots on the abdomen.
- Cowpea aphid —Cowpea aphids are black, brown, or gray-blue with white legs and antennae. About one third of the antenna end is dark, and the legs have dark “knees” and “feet.” Adults are almost 1/8 inch long (Figure 5B). The cornicles are dark and barely longer than the cauda. Nymphs are pale-green to gray with a powdery coating.
- Melon aphid—Yellow to green, melon aphids have dark cornicles and cauda and are about 1/16 inch long (Figure 5C). Wingless forms are most common. (For more information about melon aphids, see "Pests of Cucurbits.")
- Pea aphid—Long-legged, with green bodies and reddish eyes and almost 3/16 inch long, pea aphids have long, slender cornicles (Figure 5D).
- Potato aphid—Pink, mottled, or light green, potato aphids have a dark stripe down the back (Figure 5E). Adults may be a little more than 1/8 inch long, and the cornicles are slender and about twice as long as the cauda. (For more information about potato aphids, see "Pests of Lettuce.")
- Aphids—Aphids are soft-bodied, pear-shaped insects with a pair of dark cornicles (tailpipe-like appendages) and a cauda (tail) protruding from the abdomen. They may be winged or wingless, with wingless forms most common. Aphids feed in colonies and cause discoloration, curling, and deformation of foliage. They often transmit viruses, and they excrete honeydew on which sooty mold grows.
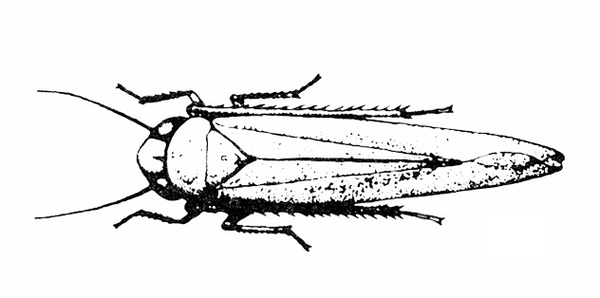
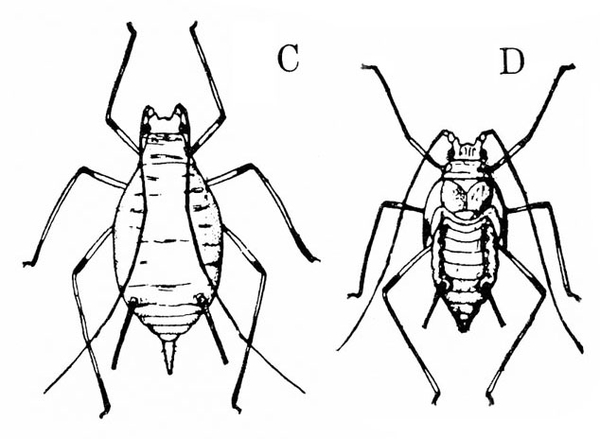
b. Potato leafhopper—Spindle-shaped and up to 1/8 inch long, potato leafhoppers are green with yellowish to dark-green spots (Figure 6). When disturbed, they usually jump rather than fly. Potato leafhoppers extract sap from the undersides of leaves, causing them to crinkle and curl downward (Figure 7). Infested plants become yellow or bronzed and dwarfed. (For more information about potato leafhoppers, see "Pests of Potato.")
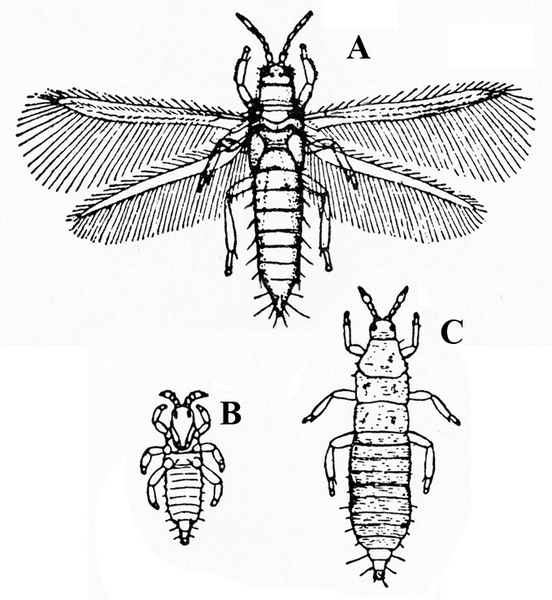
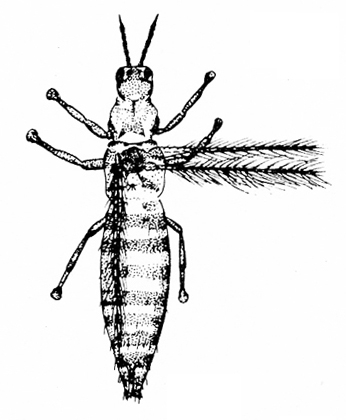
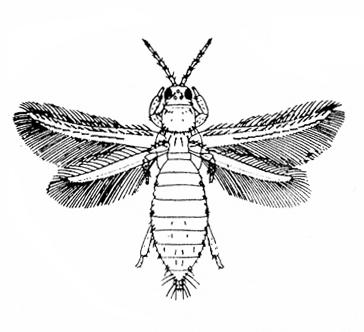
c. Thrips—Spindle-shaped and 3/32 inch or shorter in length, thrips are yellow, amber, brown, or black. Adults have two pairs of fringed wings with brown crossbands. Immature thrips are white or yellow with red eyes (Figure 8A–C). Thrips cause damage during hot, dry weather. They create whitish flecks or streaks on leaves and blossoms, and they deposit specks of black excrement.
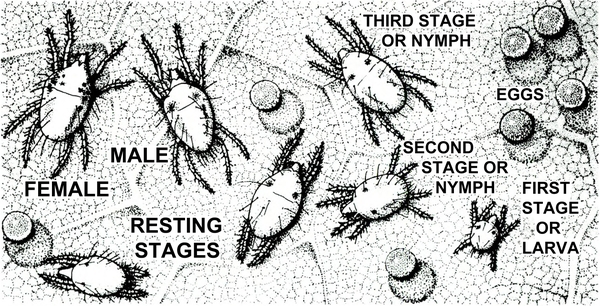
d. Twospotted spider mite—Tiny (almost microscopic) and pale to dark green, twospotted spider mites have two or four dark spots. Adults and nymphs have eight legs (Figure 9). Larvae have six legs. Females are oval and about 1/64 inch long. Males taper toward the rear. Infested foliage becomes silvery because of pale-yellow stipples. Leaves eventually become pale and die. Mites spin silken webs on the undersides of leaves as they feed.
- Insects that mine or eat holes in foliage
- Pests that feed on pods
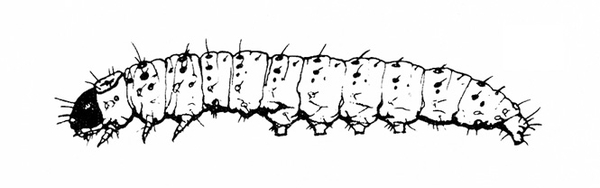
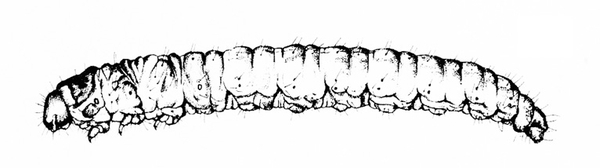
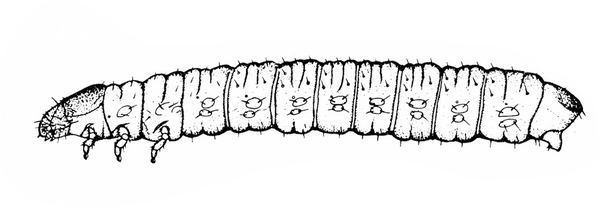
- Corn earworm—Early instars of corn earworms are cream colored or yellowish green with few markings. Later instars are green, reddish, or brown, with pale, longitudinal stripes and scattered black spots (Figure 10). Corn earworms are moderately hairy and grow to 1 3/4 inches long. They have three pairs of legs and five pairs of prolegs. They attack beans in fall, eating holes in pods. (For more information about corn earworms, see "Pests of Sweet Corn."
- European corn borer—These caterpillars are grayish pink with a dark head and rows of small, brown, doughnut-shaped spots on the back (Figure 11). European corn borers grow to about 1 inch long and bore into the pods. (For more information about European corn borers, see "Pests of Sweet Corn.")
- Cowpea curculio adult and larva (Figure 12A–B)—Adults are black, humpbacked weevils that are 1/4 inch long. Larvae are pale yellow and have brown heads. Larvae are legless and grow to 1/4 inch long. Adults leave feeding scars—small holes in pods and peas; larvae feed inside green seeds.
- Stink bugs and leaffooted bugs—Adult stink bugs are green or brown shield-shaped insects up to 3/4 inch long; nymphs are pale green or marked with orange and black (Figure 13). Leaffooted bugs are brown with wide, flat legs (Figure 14A). Leaffooted bug nymphs are red (Figure 14B). These bugs pierce buds, pods, and seeds and cause buds to be malformed and plants to be weakened. (For more information about stink bugs and leaffooted bugs, see "Pests of Okra." )
- Pests that damage seeds and roots and bore into stems
- Bean leaf beetle larva—The whitish larvae, up to 3/8 inch long, are dark at both ends and have three pairs of legs near the head (Figure 15). They cause minor damage by feeding on roots.
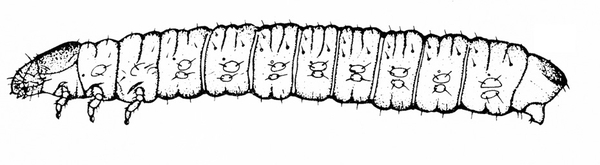
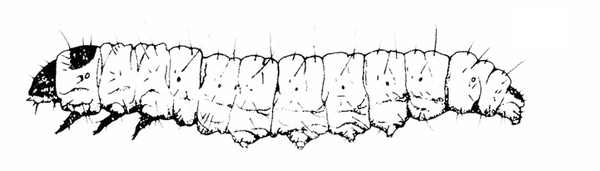
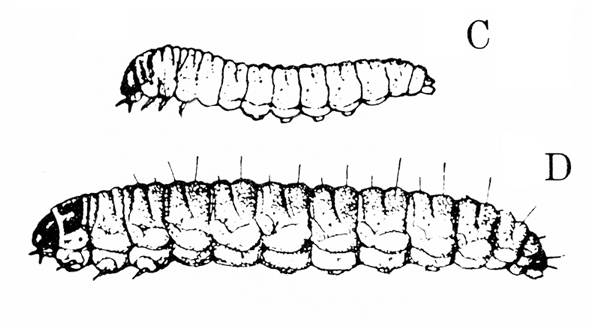
- Lesser cornstalk borer —These slender, bluish-green caterpillars are up to 3/4 inch long and have brown rings around the body, three pairs of legs near the head, and five pairs of prolegs on the abdomen (Figure 16). Young larvae bore into stems and sometimes disrupt the growing point.
- Limabean vine borer—Gray when young, these caterpillars later become bluish green and covered sparsely with long, yellowish hairs. They grow up to 1 inch long and have three pairs of legs near the head and five pairs of prolegs on the abdomen (Figure 17). Caterpillars move from the leaves into stems, usually near nodes, where they cause development of galls up to 2 3/4 inches long and 3/4 inch in circumference. Short, loose, silky frass (droppings) tubes are connected to entrance holes.
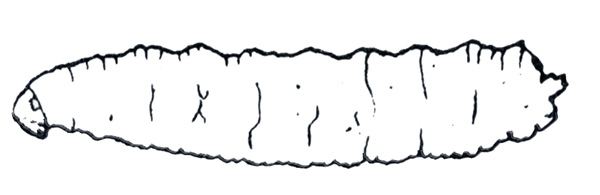
- Seedcorn maggot—These white to yellow-white, legless maggots are up to 1/4 inch long (Figure 18). The head is narrower than the body. They feed on seed contents, resulting in poor germination and tall, spindly seedlings.
Bean and Cowpea Aphids
Bean aphid, Aphis fabae Scopoli, Aphididae, HEMIPTERA
Cowpea aphid, Aphis craccivora Koch, Aphididae, HEMIPTERA
Description
Adult—These soft-bodied, pear-shaped insects have antennae that are shorter than their bodies and a pair of cornicles (tailpipe-like appendages). They may be winged (Figure 19A) or wingless, but the wingless forms are most common. The bean aphid has a dark-green to black body a little more than 1/16 inch long with white appendages (Figure 19B). The cowpea aphid (Figure 19C) has a black, brown, or gray-blue body with white legs that have black “feet” and “knees.” The antennae are white with black tips. It may grow to almost 1/8 inch long.
Egg—The egg stage probably does not occur in North Carolina.
Nymph—Though usually smaller than 1/16 inch long, nymphs resemble the wingless adults in shape. Bean aphid nymphs are green, with the last instar having five to seven pairs of white spots on the back of the abdomen. Cowpea aphid nymphs are pale-green to gray with a powdery coating.
Biology
Distribution—Bean and cowpea aphids occur in many temperate and subtropical regions of the world. In North America, the bean aphid can be found from New Brunswick to Florida and westward to California. The cowpea aphid has been reported in at least 28 scattered states and in three Canadian provinces.
Host Plants—The bean aphid infests a large number of fruiting, vegetable, agronomic, and ornamental plants, in addition to many weeds. Its vegetable hosts include asparagus, broad and lima beans, carrot, celery, corn, cowpea, cucumber, eggplant, lettuce, onion, pea, pepper, potato, spinach, tomato, and turnip. In states where winters are more severe than in North Carolina, the euonymus shrub is the primary winter host plant. In many southern states, weeds such as dock, lambsquarters, and shepherd’s-purse are favored summer hosts.
Host plants of the cowpea aphid include alfalfa, apple, carrot, cotton, cowpea, dandelion, dock, goldenrod, kidney bean, lambsquarters, lettuce, lima bean, pinto bean, peanut, pepperweed, pigweed, red clover, shepherd’s-purse, vetch, wheat, white sweet clover, and yellow sweet clover.
Damage—Congregating on lower leaf surfaces and terminal buds, aphids extract plant sap. Leaves curl and pucker, and seedling plants may become stunted and die. On lima bean, bean aphids attack terminal leaves, flower heads, and stems of pods. Infested plants develop yellow foliage, may become dwarfed and malformed, and lose vigor. A dark, sooty mold often grows on the honeydew-coated surfaces of aphid-infested plants.
Life History—Feeding and reproduction of bean aphids increase with warm weather in spring. Wingless female adults, known as "stem mothers," give birth to about 80 nymphs over a period of two and a half weeks. At a temperature of about 53°F, nymphs develop into adults in about 22 days. At a temperature of about 83°F, development takes only five days. Most nymphs mature into wingless females, but periodically, winged females develop and migrate to new host plants. Like their wingless counterparts, these adults produce offspring and thereby colonize new plants. Reproduction continues throughout the winter at a reduced rate, and many generations are produced each year. Cowpea aphids have a similar life history, though rates of development may vary.
Control
Lady beetles and their larvae, lacewing larvae, hover fly larvae, and stilt bugs feed on aphids. During periods of high humidity, fungal diseases also reduce aphid populations. If chemical control is needed, consult the North Carolina Agricultural Chemicals Manual.
Bean Leaf Beetle
Cerotoma trifurcata (Forster), Chrysomelidae, COLEOPTERA
Description
Adult—Though the adult varies greatly in color and markings, it is typically reddish-brown to yellow with black margins and about 3/16 to 1/4 inch long (Figure 20A–B). Each wing cover usually is marked with three black spots. All bean leaf beetles have a black triangular spot on the forward margin of the wings.
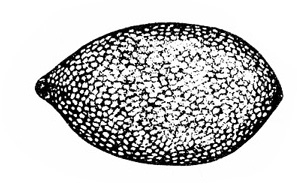
Egg—The lemon-shaped egg is orange and about 1/64 inch long (Figure 20C).
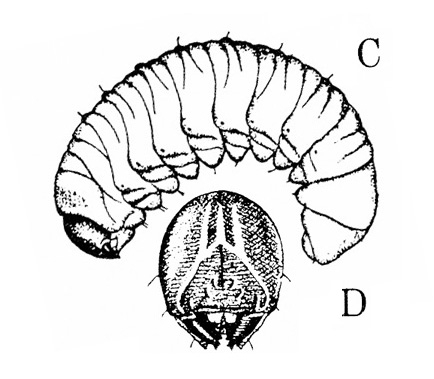
Larva—The larva is whitish, and both ends are dark brown. Conspicuously segmented, it has three pairs of legs near the head. It grows to a length of about 3/8 inch (Figure 20D).
Pupa—The pupa is exposed, white, soft-bodied, and about 3/16 inch long (Figure 20E).
Biology
Distribution—The bean leaf beetle is abundant in the southeastern states, particularly in the coastal counties. Its range, however, extends into Canada, New York, Minnesota, Kansas, Texas, and New Mexico. The insect appears to prefer poorly drained clay and organic soils.
Host Plants—Hosts of the bean leaf beetle include bean, clover, corn, cowpea, soybean, peanut, and several leguminous weeds.
Damage—Damage to bean, pea, and cowpea is caused primarily by the foliar-feeding adults. Bean leaf beetles prefer the youngest plant tissue available; when vegetative growth terminates, they will consume tender pod tissue. Pod damage is usually limited to outer layers of pod; developing beans are infrequently attacked. In North Carolina, these beetles damage leaves and stems from late May through September. In addition to the beetles' direct attack, adults are also known vectors of the bean pod mottle, cowpea mosaic, and southern bean mosaic viruses. Bean leaf beetle larvae do a little damage by feeding on roots.
Life History—Adults overwinter in leaf litter or other vegetation, primarily in wooded areas. They become active in April and move to emerging host plants. In the southeastern United States, beetles usually do not attack beans or peas until mid-May. They feed voraciously for several days and then mate. Each female lays 175 to 250 eggs in clusters of 12 to 24 in the soil at the plant's base. Eggs hatch in one to three weeks, depending upon temperature. Larvae find their way to the base of the stem or roots and feed there for three to six weeks. Mature larvae form earthen cells within which the pupae form. In southern states, peak periods of adult activity generally occur the last of May, the last of July, the second and third weeks of August, and the second and third weeks of September. Second-generation beetles overwinter in North Carolina.
Control
Chemical control consists of applying foliar insecticides or using a granular insecticide in-furrow at planting. Control practices for the Mexican bean beetle also control the bean leaf beetle. For up-to-date recommendations, consult the North Carolina Agricultural Chemicals Manual.
Cowpea Curculio
Chalcodermus aeneus Boheman, Curculionidae, COLEOPTERA
Description
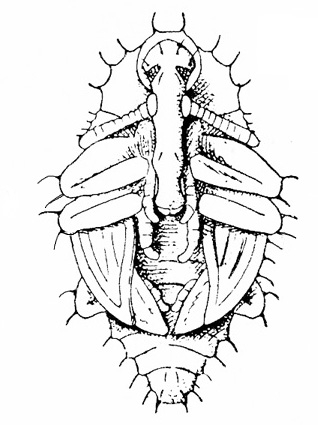
Adult—This weevil is also referred to as the black humpbacked snout beetle. The adult is black and humpbacked, slightly tinged with bronze, and 1/4 inch long (Figure 21A and Figure 22).
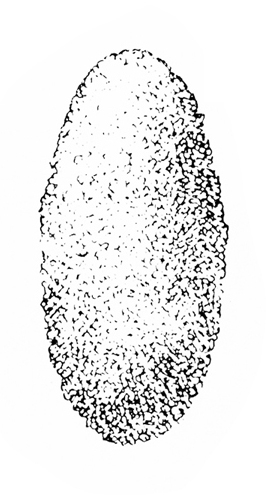
Egg—Each oval egg is about 1/16 inch long (Figure 21B). Translucent when first laid, it becomes opaque and white before hatching.
Larva—This legless grub is pale yellow with a brown head (Figure 21C–D). It is about 1/4 inch long when fully grown.
Pupa—The pale-yellow pupa, about 3/16 inch long, darkens as it matures (Figure 21E). Its shape is similar to that of the adult.
Biology
Distribution—In the United States, the cowpea curculio is most common throughout the South Atlantic and Gulf Coast states. It has been reported as far north as Maryland and Iowa, as far west as Texas and Oklahoma, and as far south as Mexico and parts of South America.
Host Plants—The cowpea curculio infests field peas, snap bean, soybean, lima bean, cotton, and strawberry. Black-eyed pea and crowder pea are most commonly attacked. Several leguminous weeds, including vetch, are also hosts.
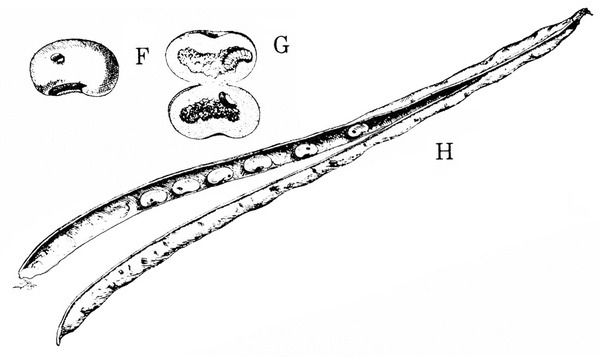
Damage—Female weevils disfigure the pods of legumes with their oviposition (egg-laying) holes. The larvae ruin the seeds inside as they feed on them and grow (Figure 21F–H).
Life History—Cowpea curculio adults pass the winter in crop refuse or weeds, particularly brown sedge, around previously infested plants. They leave their overwintering sites from April through July. Weevils puncture developing pods with their snouts as they feed. The female lays a single egg in a feeding wound (and sometimes directly into the bean). About four days later, a brown-headed grub hatches and infests the seeds of beans and peas. After feeding for two or three weeks, grubs chew exit holes through the pods, drop to the ground, dig into the soil, and pupate. All grubs usually leave the pods within seven days of one another. About 10 days later, a new generation of adults emerges. There are two overlapping generations each year in North Carolina.
Control
The cowpea curculio is a difficult pest to control because it has acquired resistance to pyrethroids and other insecticides and it migrates into fields by crawling and flying. Tillage of weed hosts and crop residue helps destroy overwintering beetles. Insecticides are still needed for acceptable control, and a synergist should be used at the high rate with pyrethroids. For efficient scheduling of integrated pest management (IPM) practices and insecticide applications, consult your local N.C. Cooperative Extension center.
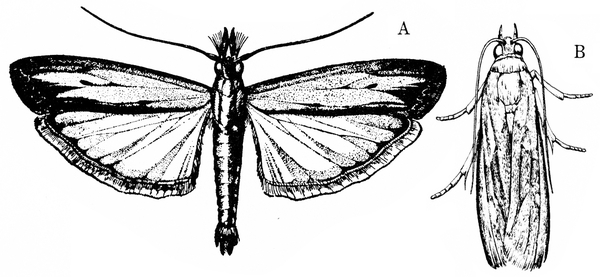
Lesser Cornstalk Borer
Elasmopalpus lignosellus (Zeller), Pyralidae, LEPIDOPTERA
Description
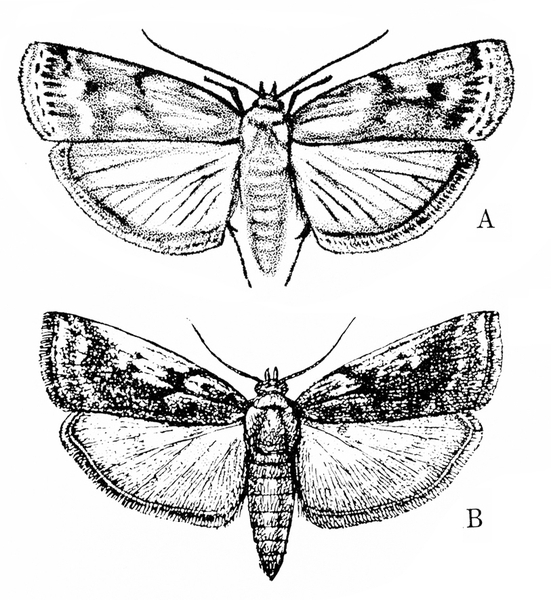
Adult—The moth has a wingspan of nearly 1 inch (Figure 23A–B). The male's front wings are brownish yellow and have grayish margins with several dark spots. The female's front wings are nearly black.
Egg—The egg is greenish white and less than 1/16 inch in diameter (Figure 23C).
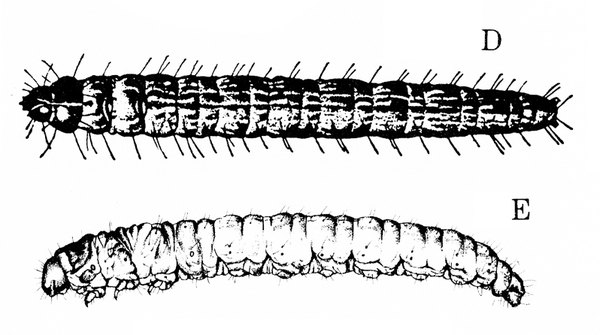
Larva—The larva is a slender, bluish-green, brown-striped caterpillar about 3/4 inch long (Figure 23D–E)
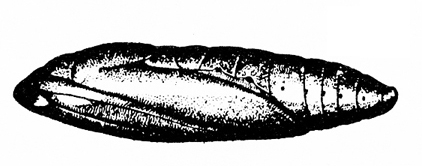
Pupa—The pupa is brownish and about 3/8 inch long (Figure 23F).
Biology
Distribution—Though the lesser cornstalk borer is found from Maine to Southern California, the bulk of its damage occurs in the southern states, particularly Alabama, Georgia, and South Carolina. It is also found in Mexico, Central America, and South America.
Host Plants—The lesser cornstalk borer prefers corn and legumes, but it also feeds on bean, cowpea, crabgrass, johnsongrass, pea, peanut, sorghum, soybean, and wheat.
Damage—Larvae of this small moth have been sporadically injurious to seedlings of many plant species, and this pest seems to be on the increase in the South. Injury is caused when a larva bores into the stalk of a host plant, thereby disrupting the growing point. Damage can be slight, or the plant can die. Damage is most prevalent in crops grown on sandy soils during dry conditions.
Life History—Lesser cornstalk borers hibernate as larvae or pupae. In North Carolina, they usually overwinter as larvae, which develop into pupae before spring. Moths emerge in early spring and lay eggs on or near the host's leaves or stems. Eggs hatch in two to seven days. On peas, the larvae tunnel up succulent main stems, causing them to wilt. They can seldom penetrate older stems. Later they construct underground silken tubes or burrows from which they bore into plants near the ground level. They become fully grown in two to three weeks, leave their burrows, and spin silken cocoons under trash on the surface of the ground. In these cocoons, they change to pupae, emerging as moths in two to three weeks. Two generations are known to occur in the mid-Atlantic states; three generations occur in South Carolina; and four generations occur in southern Georgia and Florida.
Control
Few natural enemies are successful at controlling the lesser cornstalk borer in dry spells. Lesser cornstalk borer larvae are well adapted to dry conditions. Avoid planting in dry soils or fields that have been treated with herbicides less than two weeks prior. Fall plowing helps kill overwintering borers. Early planting also helps keep their populations in check. Conservation tillage can also lessen plant injury because when crop residue remains at the soil surface, the larvae feed on the residue instead of the newly seeded plants. Keeping the soil moist through irrigation can also deter the females from laying their eggs. Satisfactory control is often dependent on chemical methods. Granular insecticides are often applied in the seed furrow to control this pest. Liquid pesticides can work also, but they must be aimed at the plants' roots. After applying insecticides, irrigate the treated area to increase insect control. For up-to-date chemical recommendations, consult the North Carolina Agricultural Chemicals Manual.
Limabean Vine Borer
Monoptilota pergratialis (Hulst), Pyralidae, LEPIDOPTERA
Description
Adult—This brownish-gray moth has whitish scales on the edge and across the end of its forewings. The forewings are marked with small black streaks and are black along the veins. Hind wings and the outer edges of the forewings have a brownish-gray, fringe-like border (Figure 24A–B). The male’s hind wings are white, whereas the female’s are more grayish. The wingspan is about 3/4 inch.
Egg—The dull-gray, oblong-oval egg is almost 1/32 inch long and 1/64 inch wide.
Larva—Gray when very small, the larva gradually becomes bluish green and sparsely covered with long, yellow hairs (Figure 24C–D). Behind the black or brown head capsule is a yellowish-brown prothoracic shield. When fully grown, this caterpillar is 3/4 to 1 inch long.
Pupa—The yellowish to reddish-brown pupa is 1/2 inch to 9/16 inch long and is enclosed in a 5/8-inch-long cocoon (Figure 24E).
Biology
Distribution—Although this pest occurs throughout much of the east central and southeastern United States, it is primarily a problem along the coastal plain from Delaware and Maryland south to Florida and west to Alabama. The limabean vine borer also occurs in some southwestern states, including Arizona.
Host Plants—This pest heavily infests pole and lima beans. Though its occurrence on other hosts is rare, this caterpillar will feed on snap bean, cowpea, and dahlia. It prefers large-stemmed bean varieties.
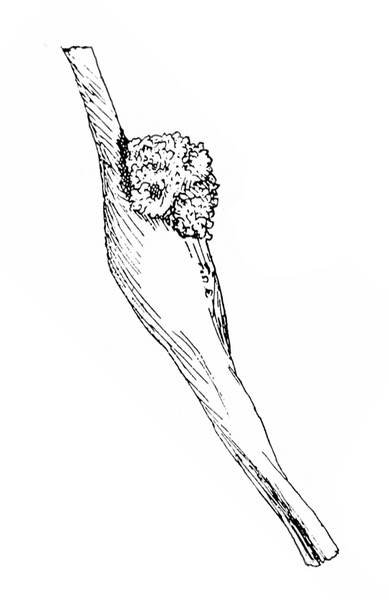
Damage—Young larvae feed on leaves, slightly skinning the lower epidermis and leaving behind telltale frass (droppings) or webbing (Figure 24F). As larvae mature, they bore into stems, typically just above or below nodes, and hollow out cavities (Figure 25). As a result, infested stems gradually swell and form galls up to 2 3/4 inches long and 3/4 inch in circumference (Figure 25). The galls eventually turn brown and develop a woody texture. Short, loose, silky frass tubes are attached to the entrance holes on the galls. Usually, infested plants are weakened and have lower yields. When galls are located near the tips of small stalks, the tops of plants often wilt, and full-size pods cannot be produced. The extent of damage varies with the position of the gall and the vigor of the host plant.
Life History—Limabean vine borers overwinter as prepupae on or near the soil surface. The prepupa is a developmental stage in which the larva shrivels slightly but is able to delay development until the following spring, at which time it pupates. Moths emerge from late April to mid-May in eastern North Carolina and deposit eggs on leaves or in naturally occurring depressions in the stems of host plants. Two to six days later, eggs hatch, and larvae begin feeding on leaves. After feeding for four to seven days, larvae bore into stems, where they continue feeding and complete their development. When fully grown, larvae bore exit holes in the galls, drop to the ground, and enter the soil, where they spin cocoons and pupate. A new generation of moths emerges about 15 days later. Larvae usually can be found from May through October. Later in the season, larvae may take over galls formed by previous generations of larvae instead of creating their own. In North Carolina, about three generations occur each year.
Control
Hand removal of galls does more harm than good to infested plants. Fall plowing or winter cultivation help reduce populations by destroying overwintering prepupae. Where practical, crop rotation is recommended. Foliar sprays to control this pest should begin when pods start to form. For up-to-date chemical recommendations, consult the North Carolina Agricultural Chemicals Manual.
Mexican Bean Beetle
Epilachna varivestis Mulsant, Coccinellidae, COLEOPTERA
Description
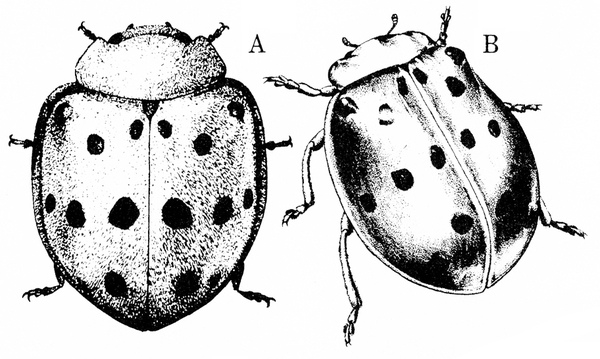
Adult—This copper-red beetle is 1/4 to 5/16 inch long and dome shaped (Figure 26A–B). Overwintering beetles are lighter in color. Each wing cover has eight small, black spots that form three rows across the body when the wings are at rest.
Egg—The yellow egg is almost 1/16 inch long and elliptical.
Larva—The mature larva is about 5/16 inch long, yellow, and covered with dark, branched spines (Figure 26D–E).
Pupa—The yellow to copper-colored pupa is about 1/4 inch long and has fewer spines than the larva (Figure 26F). It moves very little.
Biology
Distribution—Formerly, the Mexican bean beetle was limited to Colorado southward. It is now common throughout the United States with the exception of the Pacific Coast states.
Host Plants—Mexican bean beetles have a wide variety of hosts but are most commonly encountered on garden and field beans as well as cowpea. They may also attack soybean, clover, alfalfa, and closely related plants.
Damage—The Mexican bean beetle is the most injurious pest of beans, including snap, lima, pole, kidney, pinto, navy, and bush. If overwintering populations are high, seedlings may be injured, though economically significant damage usually does not occur before August. Larvae and adults feed on the undersides of leaves, leaving the upper surface intact. Damaged plants have a characteristic lacelike (skeletonized) appearance. These remaining tissues die within about two days and turn brown, often giving the entire field a "burnt" cast. Pods and stems are often attacked, and heavily damaged plants may die before any crop matures.
Life History—Adult beetles overwinter in hedgerows, ditch banks, and woodlands and may attack plants soon after seedlings emerge in spring. Most beetles leave their winter quarters over a two-month period. After feeding, adult females deposit eggs in clusters of 40 or more on the undersides of leaves (Figure 26C). Eggs hatch in five to fourteen days, and larvae feed for two to five weeks. Larvae pupate on leaves, and adults emerge after about 10 days. Adults feed, mate, and lay eggs over a period of two weeks. The time from egg to adult is about 30 days. In North Carolina, there are three or four generations each year (Figure 27).
Control
The snap bean varieties Wade, Logan, and Black Valentine are generally less severely damaged by the Mexican bean beetle than other varieties. Because damage is usually most severe during July and August, very early-maturing bean varieties and fall plantings may be grown with little injury. Destroying crop residue helps prevent population buildup. A parasite, Pediobius foveolatus, is commercially available for managing Mexican bean beetles. Chemical control includes applying foliar insecticides to the undersides of leaves or using a granular insecticide in-furrow at planting. For up-to-date recommendations, consult the North Carolina Agricultural Chemicals Manual.
Pea Aphid
Acyrthosiphon pisum (Harris), Aphididae, HEMIPTERA
Description
Adult—The adult pea aphid is long-legged and light-to-deep-green with reddish eyes. It can have a body length of 1/16 inch to almost 3/16 inch. A winged adult may be as long as 3/16 inch from its head to the tip of its wings (Figure 28A–B). Cornicles (a pair of tailpipe-like structures projecting from the abdomen) of this aphid are characteristically long and slender. Wingless pea aphids are pale green with pale-amber legs and cornicles.
Egg—About 1/32 inch long, the light-green egg turns shiny black before hatching. The egg stage does not occur in North Carolina.
Nymph—Immature pea aphids are usually smaller than but similar to the larger wingless adult (Figure 28C–D). Nymphs destined to be winged adults develop wing buds as they mature. They require four molts to reach the adult stage.
Biology
Distribution—The pea aphid is found throughout the United States and Canada wherever peas and alfalfa are grown.
Host Plants—Pea aphids infest garden peas, field peas, sweet peas, sweet clover, alfalfa, and some leguminous weeds. Vetch and crimson clover are important overwintering hosts.
Damage—Pea aphids extract sap from the terminal leaves and stem of the host plant. They also feed on pods, causing them to curl, shrink, and fill only partially. Their feeding can result in deformation, wilting, or death of the host, depending upon the infestation level and size of the plant. Plants under 6 inches tall may be killed by a few aphids, whereas larger plants may be only slightly damaged. Infested plants are often coated with shiny honeydew secreted by aphids, and shed aphid skins may give leaves and the ground a whitish appearance. In North Carolina, these aphids transmit yellow bean mosaic virus.
Life History—In North Carolina, wingless female pea aphids continue to feed and breed throughout the winter. Aphid activity increases in spring. Each adult female gives birth to 10 to 14 nymphs each day until she has produced about 100 offspring. Nymphs mature into adults in 10 to 14 days. Most nymphs develop into wingless female adults. When overcrowding occurs, however, winged aphids develop, migrate to other host plants, and establish new colonies. Because generations overlap and reproduction continues all year, the number of annual generations is difficult to determine. The pea aphid thrives best and reproduces most rapidly at a temperature of about 65°F and humidity near 80 percent.
Control
For up-to-date chemical control recommendations, consult the North Carolina Agricultural Chemicals Manual.
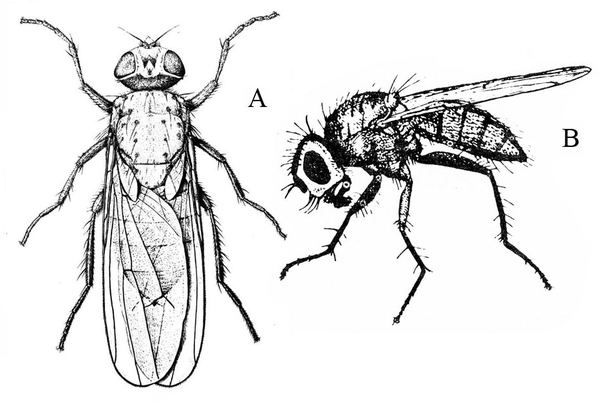
Seedcorn Maggot
Delia platura (Meigen), Anthomyiidae, DIPTERA
Description
Adult—This gray, black-legged fly has scattered bristles on its body and is about 3/16 inch long (Figure 29A–B).
Egg—Each white, elongate egg has a rough surface and is almost 1/16 inch in length.
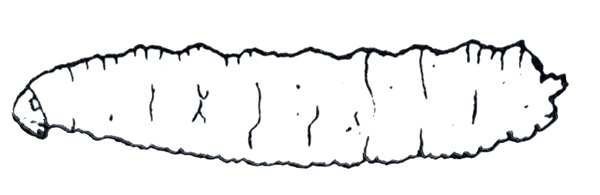
Larva—This 12-segmented, white to yellow-white maggot is 3/16 to 1/4 inch long when mature. It is legless and tough-skinned with a pointed head and rounded tail (Figure 29C).
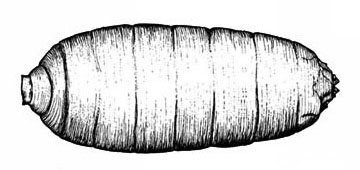
Puparium—The skin of the mature larva hardens to form a puparium about 3/16 inch long in which the pupa develops (Figure 29D). The ivory puparium gradually turns reddish brown as the pupa matures.
Biology
Distribution—Common throughout the temperate zones of the world, the seedcorn maggot is found in all arable portions of North America from southern Canada into Mexico. It has not been found at altitudes above 4,500 feet. This pest is usually a problem during cold, wet seasons and on land with high levels of organic matter.
Host Plants—Although it feeds primarily on decaying organic matter, the seedcorn maggot infests roots or seeds of more than 47 kinds of plants. In North Carolina, it is a pest of bean, pea, cucumber, melon, onion, corn, pepper, and potato.
Damage—Seedcorn maggots damage newly planted seeds by feeding on seed contents, often leaving empty shells and resulting in poor germination. Infested seedlings that emerge are tall and spindly with few leaves; these rarely mature or mature late because of damage. Occasionally, seedcorn maggots tunnel into seedling stems (Figure 30). Either type of feeding allows entry of disease-causing organisms.
Life History—In North Carolina, all stages of the seedcorn maggot (Figure 30) can be found throughout the winter. Farther north, however, these insects overwinter in the soil as pupae. Adult flies emerge from puparia at night or early in the morning and push themselves up to the soil surface. Adults feed on nectar and honeydew. Females lay an average of 270 eggs, singly or in small clusters, in moist soil. Freshly distributed soil, fields with decaying seed or crop remnants, and soils rich in organic matter are highly attractive to ovipositing (egg-laying) female flies.
Eggs hatch in seven to nine days. Newly hatched maggots tunnel into seeds or decaying vegetable matter. Maggots remain active at temperatures as low as 40°F. They develop through three larval stages. After feeding for one to three weeks, the larvae burrow as deep as 7 inches into the soil to pupate. Pupation may last seven to twenty-six days or all winter.
In the eastern United States, three to five generations of seedcorn maggots develop each year. Generations that occur during spring and fall are the most abundant and destructive. During summer, a limited number of adults survive.
Control
Seeding shallowly in a well-prepared bed and planting sufficiently late for quick germination may prevent injury by seedcorn maggots in field or vegetable crops. Land where manure is heavy or where a cover crop is to be turned under should be plowed early in the fall, if possible; this renders the field less attractive to egg-laying flies the following spring. Prompt resetting or replanting of damaged crops usually ensures a good stand.
Combination fungicide-insecticide seed treatments or soil-applied insecticides can prevent seedcorn maggot damage. For up-to-date chemical recommendations, consult the North Carolina Agricultural Chemicals Manual.
Thrips
Flower thrips, Frankliniella tritici (Fitch), Thripidae, THYSANOPTERA
Soybean thrips, Sericothrips variabilis (Beach), Thripidae, THYSANOPTERA
Tobacco thrips, Frankliniella fusca (Hinds), Thripidae, THYSANOPTERA
Description
Adult—Thrips are about 1/16 inch long and have two pairs of long, narrow wings fringed with long hairs. The flower thrips (Figure 31A) is yellowish-brown to amber, with an orange thorax. The soybean thrips (Figure 31B) has a yellow body with a dark blotch on the thorax and two distinct crossbands on the forewings. The tobacco thrips (Figure 31C) is dark brown or black.
Egg—Thrips eggs are about 1/64 inch long, cylindrical, and slightly kidney-shaped, with a smooth white or yellow surface.
Larva—The two larval instars are about 1/16 inch long. Larvae are white when newly hatched, then gradually turn yellow with age. The soybean thrips larva eventually turns orange with some red pigmentation, though the body sometimes has a greenish tint due to ingested chlorophyll.
Prepupa and Pupa—Pupal stages resemble larvae in shape and color, but prepupae have short wing pads, whereas pupae have long wing pads. The prepupa is almost 1/16 inch long and shrinks slightly as it becomes a pupa. The pupa usually remains motionless unless disturbed.
Biology
Distribution—These three thrips species are common throughout the eastern United States. The soybean thrips also occurs in California, Arizona, Utah, and Texas. The flower thrips migrates in frontal wind systems, especially in June.
Host Plants—Flower and tobacco thrips have been collected from many plant orders, including a wide range of flowers, trees, grasses, field crops, vegetables, vines, and weeds. Flower thrips seem to prefer grasses and yellow or other light-colored blossoms. Soybean thrips feed primarily on soybean, bean, and other legumes but also infest cotton, cucumber, smartweed, and a number of grasses.
Damage—Thrips are relatively minor pests in North Carolina. They feed on the undersides of bean and soybean leaves (Figure 31D) throughout the growing season, reaching maximum densities about a month after planting. Six to ten thrips per leaf may cause some yellowing but relatively little economic damage. Early-season drought may cause an ordinarily harmless thrips population to become a problem. Thrips damage occurs primarily during periods of vegetative growth and is difficult to distinguish from damage inflicted by a wide range of pests and diseases that cause yellowing and browning of leaves in late summer. On soybeans, populations of 30 to 60 thrips per leaf have been reported to cause substantial injury.
Life History—In North Carolina, thrips overwinter as hibernating adults in sheltered areas, as larvae on plants, or as pupae in soil. They resume development in spring. Adults emerge and fly in search of suitable host plants. Though weak flyers, adult thrips are capable of flying from plant to plant, and they may be carried long distances by wind.
The maximum number of eggs laid by each female thrips has not been determined, but most species of thrips insert up to 100 eggs, singly, into leaves and succulent stems. About four days later, eggs hatch. At a temperature of 72°F, larvae feed for about seven days on the undersides of leaves, often hiding near large leaf veins. Mature second-instar larvae drop to the soil and make chambers in the center of small dirt clods. Here, they turn into quiescent, nonfeeding prepupae. Soon prepupae transform into pupae. About three days later, a new generation of adults emerges. Most thrips species complete five or more generations in North Carolina.
Control
Several predators can limit the size of thrips populations—including a cocoon-spinning thrips, Aeolothrips fasciatus (Linnaeus), which is about 1/16 inch long, dark as an adult, and yellow as a larva; the insidious plant bug, Orius insidiosus (Say), a tiny chinch-bug-like insect; and several phytoseiid mites. For up-to-date chemical recommendations, consult the North Carolina Agricultural Chemicals Manual.
Twospotted Spider Mite
Tetranychus urticae Koch, Tetranychidae, PROSTIGMATA
Description
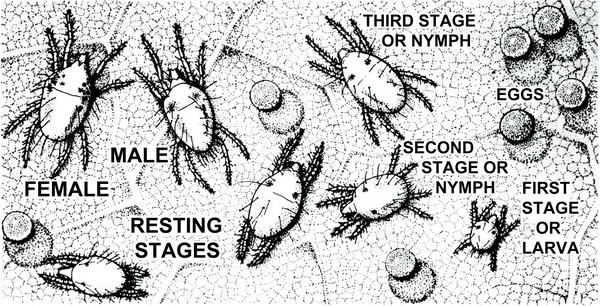
Adult—The eight-legged adult is almost microscopic. Oval in shape, the summer female is yellowish to dark-green with two or four dark dorsal spots. Smaller and more active than the female, the male has a narrower body and a more pointed abdomen.
Egg—The egg is spherical, minute, and transparent when first deposited. It gradually assumes a yellowish-green color.
Larva—The six-legged larva, not much bigger than the egg, is colorless except for carmine eye spots.
Nymph—The two nymphal stages are difficult to distinguish. Both are pale green, oval, and eight-legged. A pair of dark spots on the abdomen is usually visible at this stage of development.
Biology
Distribution—Twospotted spider mites are widely distributed throughout the world. In the southeastern United States, they are pests throughout the coastal plain.
Host Plants—Twospotted spider mites have been found on more than 180 host plants, including at least 100 cultivated species. Violets, chickweed, pokeweed, wild mustard, hairy vetch, red clover, Carolina geranium, and blackberry are common hosts from which infestations spread to nearby cultivated crops.
Damage—Mites pierce the epidermis and extract sap from the undersides of leaves. Infested foliage soon assumes a whitish or bronze appearance. Lightly infested leaves have pale blotches or spots showing through the leaf; heavily infested leaves turn completely pale and dry up. The undersides of leaves usually are covered with silken webs over which the mites crawl. Heavily infested plants may have webs all over them. Close examination reveals adult mites on the leaves.
Life History—Twospotted spider mites (Figure 32) overwinter as females tolerant of low temperatures. These females are reddish, while the active summer forms are yellowish green. In greenhouses or during mild winters, some feeding and egg-laying activities may continue.
In warm weather, egg-laying activities increase; each female produces as many as 19 eggs per day and as many as 100 eggs in all. The number of eggs laid depends largely on temperature. After an incubation period of three to nineteen days, six-legged larvae hatch from the eggs and feed. Immature mites go through a resting period between the larval and nymphal stages and again after each of the two nymphal instar stages are complete. The mites may mature into adults in as few as five days or as many as twenty, depending on temperature. Development is most rapid during hot, dry weather. Many generations are produced each year. Spider mites are distributed over a field via females crawling, or they are carried from infested areas by wind, animals, people, and farm implements.
Control
Destroying weeds around the field in fall or early spring is a cultural practice that reduces overwintering mites. Destroying weeds along fencerows or around the edges of fields during the growing season is not advisable, however, as it forces mites to migrate into the field.
Several miticides provide effective chemical control. A second application five to seven days after the first is often advised. For recommended miticides and rates, consult the North Carolina Agricultural Chemicals Manual.
Other Resources
General
Harris, Jr., E. D. Insect Control on Commercial Beans and Southern Peas. Circular 567. University of Georgia Cooperative Extension Service, 1973.
Singh, S. R., and H. F. van Emden. “Insect Pests of Grain Legumes.” Annual Review of Entomology 24 (1979): 255–78.
Bean Aphid
Fraser, R. D. “Life Tables and Intrinsic Rates of Increase of Apterous Black Bean Aphids and Pea Aphids on Broad Bean.” The Canadian Entomologist 104 (1972): 1717–22.
Jones, M. G. “The Summer Hosts of Aphis fabae Scop.” Bulletin of Entomological Research 33 (1942): 161–69.
Kennedy, J. S., and C. O. Booth. “Host Alternation in Aphis fabae Scop.” Annals of Applied Biology 41 (1954): 88–106.
Lowe, H. J. B., and S. J. Rolfe. “A Key to Immature Forms of Aphis fabae.” Plant Pathology 23 (1974): 162–63.
Osborn, H., and E. A. Sirrine. “Life History of a Common Plant Louse, Aphis rumicis L.” Iowa Agricultural Experiment Station Bulletin 23 (1893): 901–5.
Way, M. J. “The Nature and Causes of Annual Fluctuations in Numbers of Aphis fabae Scop. on Field Beans.” Annals of Applied Biology 59 (1967): 175–88.
Bean Leaf Beetle
Bioteau, G., J. R. Bradley, Jr., and J. W. Van Duyn. “Bean Leaf Beetle: Flight and Dispersal Behavior.” Annals of the Entomological Society of America 72 (1979a): 298–302.
Bioteau, G., J. R. Bradley, Jr., and J. W. Van Duyn. “Bean Leaf Beetle: Some Seasonal Anatomical Changes and Dormancy.” Annals of the Entomological Society of America 72 (1979b): 303–7.
Boiteau, G., J. R. Bradley, Jr., and J. W. Van Duyn. “Bean Leaf Beetle: Emergence Patterns of Adults from Overwintering Sites.” Environmental Entomology 8 (1979c): 427–31.
Britton, W. E. “The Bean Leaf Beetle Cerotoma trifurcata Forst.” Connecticut Agricultural Experiment Station Bulletin 211 (1918): 327–29.
Eddy, O. O., and W. C. Nettles. The Bean Leaf Beetle. Bulletin 265. South Carolina Agricultural Experiment Station, 1930.
Isley, D. 1930. The Biology of the Bean Leaf Beetle. Bulletin 248. Arkansas Agricultural Experiment Station, 1930.
Cowpea Aphid
Dransfield, B., and B. Brightwell. Aphis craccivora. Cowpea Aphid, Groundnut Aphid. InfluentialPoints.com, n.d.
Cowpea Curculio
Arant, F. S. Life History and Control of the Cowpea Curculio. Bulletin 246. Alabama Agricultural Experiment Station, 1938.
Capinera, J. L. Common Name: Cowpea Curculio. Scientific Name: Chalcodermus aeneus Boheman (Insecta: Coleoptera: Curculionidae). Featured Creatures. Publication Number: EENY–223. Entomology and Nematology, FDACS/DPI, EDIS, University of Florida, 2017.
Dupree, M., and C. M. Beckham. “The Cowpea Curculio—a Pest of Southern Field Peas.” Georgia Agricultural Experiment Station Bulletin 6 (1955): 1–32.
Harris, Jr., E. D. The Cowpea Curculio. Leaflet 56. University of Georgia Cooperative Extension Service, 1966.
Hetrick, L.A. The Cowpea Curculio: Its History and Control. Bulletin 409. Virginia Agricultural Experiment Station, 1947.
Majumdar, A., et al. Cowpea Curculio Management in Alabama. ANR-2313. Alabama Extension, Alabama A&M & Auburn Universities, 2018.
Russell, C. E., and R. B. Chalfant. “An Evaluation of Worthmore, a New Variety of Southern Pea, for Resistance to the Cowpea Curculio, Chalcodermus aeneus Boh.” Journal of the Georgia Entomological Society 14 (1979): 155–57.
Lesser Cornstalk Borer
All, J. N., and R. N. Gallaher. “Detrimental Impact of No-Tillage Corn Cropping Systems Involving Insecticides, Hybrids, and Irrigation on Lesser Cornstalk Borer Infestations.” Journal of Economic Entomology 70 (1977): 361–65.
Dupree, M. “Insecticidal and Cultural Control of the Lesser Cornstalk Borer.” In Mimeograph Series N.S. 197, 21. Georgia Agricultural Experiment Station, 1964.
Dupree, M. “Observations on the Biology of the Lesser Cornstalk Borer.” Journal of Economic Entomology 58 (1965): 1156–57.
Leuck, D. B. “Biology of the Lesser Cornstalk Borer (Elasmopalpus lignosellus) in South Georgia.” Journal of Economic Entomology 59 (1966): 797–801.
Luginbill, P., and G. G. Ainslie. The Lesser Cornstalk Borer. Bulletin 539. USDA, 1917.
Limabean Vine Borer
Branon, L. W. “Biology and Control of the Lima Bean Vine Borer.” Journal of Economic Entomology 38 (1945): 407–8.
Chittenden, E. H. “A New Vine-Borer of Lima Beans, Monoptilota nubilella Hulst.” In Some Insects Injurious to Garden Crops (Bulletin 23), 9–17. U.S. Department of Agricultural Division of Entomology, 1900.
Neunzig, H. H. “Monoptilota pergratialis (Hulst).” In Systematics of Immature Phycitines Associated with Leguminous Plants in the Southern United States (Technical Bulletin 1589), 23–26. USDA, 1979.
Mexican Bean Beetle
Brown, A., and R. Hazzard. Mexican Bean Beetle, Biological Control. Center for Agriculture, Food, and the Environment, UMass Extension Vegetable Program, University of Massachusetts Amherst, 2021.
Crawford, J. C. “The Mexican Bean Beetle in North Carolina.” North Carolina Department of Agriculture Bulletin 45, no. 11 (1924): 1–12.
Harris, Jr., E. D. Mexican Bean Beetle. Leaflet 85. University of Georgia Cooperative Extension Service, 1976.
Howard, N. F., and L. L. English. Studies of the Mexican Bean Beetle in the Southeast. Bulletin 1243. U.S. Department of Agriculture, 1924.
Thomas, L. F. Life History and Control of the Mexican Bean Beetle. Bulletin 221. Alabama Agricultural Experiment Station, 1924.
U.S. Department of Agriculture. Controlling the Mexican Bean Beetle. Leaflet 548. USDA, 1974.
U.S. Department of Agriculture. The Mexican Bean Beetle in the East and Its Control. Farmers' Bulletin 1624. USDA, 1960.
Pea Aphid
Dudley, J. E., and T. E. Bronson. The Pea Aphid on Peas and Methods for Its Control. Farmers' Bulletin 1945. USDA, 1952.
Harper, A. M., et al. The Literature of Arthropods Associated with Alfalfa: III. A Bibliography of the Pea Aphid, Acyrthosiphon pisum (Harris) (Homoptera: Aphididae). Special Publication 50. Illinois Agricultural Experiment Station, 1978.
Smith, C. F. Pea Aphid Control in North Carolina. Special Circular No. 7. North Carolina Agricultural Experiment Station, 1948.
Seedcorn Maggot
Elmore, J. C. The Seed-Corn Maggot on Beans: How to Control It. Leaflet 370. USDA, 1962.
Miller, L. A., and R. J. McClanahan. “The Life History of the Seed-Corn Maggot, Hylemya cilicrura (Bond) and of H. liturata (Mg) (Diptera: Anthomyiidae) in Southwestern Ontario.” The Canadian Entomologist 92 (1960): 210–21.
Reid, Jr., W. J. Biology of the Seed-Corn Maggot in the Coastal Plain of the South Atlantic States. Technical Bulletin 723. USDA, 1940.
Vea, E. V., D. R. Webb, and O. J. Eckenrode. Seedcorn Maggot Injury. New York’s Food and Life Sciences Bulletin no. 55 (1975): 1–3.
Vea, E. V., and O. J. Eckenrode. “Resistance to Seedcorn Maggot.” Environmental Entomology 5 (1976a): 735–37.
Vea, E. V., and O. J. Eckenrode. “Seedcorn Maggot Injury on Surviving Bean Seedlings Influences Yield.” Journal of Economic Entomology 69 (1976b): 545–47.
Thrips
Bailey, S. R. “The Distribution of Injurious Thrips in the United States.” Journal of Economic Entomology 33 (1940): 133–36.
Hinds, W. E. “Monograph of the Thysanoptera of North America.” In Proceedings of the United States Natural Museum 26, 1310 (1902): 79–242.
Irwin, M. E., K. V. Yeargan, and N. L. Marston. “Spatial and Seasonal Patterns of Phytophagous Thrips in Soybean Fields with Comments on Sampling Techniques.” Environmental Entomology 8 (1979): 131–40.
Mueller, A. J., and R. G. Luttrell. “Thrips on Soybeans.” Arkansas Farm Research 26, no. 4 (1977): 7.
Robinson, A. G., L. J. Stannard, Jr., and E. J. Armbrust. “Observations on Predators of Sericothrips variabilis Beach (Thysanoptera).” Entomological News 83 (1972): 107–11.
Vance, T. C. “Larvae of the Sericothripini (Thysanoptera: Thripidae), with Reference to Other Larvae of the Terebrantia of Illinois.” Illinois Natural History Survey Bulletin 31 (1974): 141–208.
Watts, J. G. A Study of the Biology of the Flower Thrips Frankliniella tritici (Fitch) with Special Reference to Cotton. Bulletin 306. South Carolina Agricultural Experiment Station, 1936.
Twospotted Spider Mite
Huffaker, C. B., M. van de Vrie, and J. A. McMurtry. “The Ecology of Tetranychid Mites and Their Natural Control.” Annual Review of Entomology 14 (1969): 125–74.
Laing, J. E. “Life History and Life Tables of Tetranychus urticae Koch.” Acarologia 11 (1969): 32–42.
McMurtry, J. A., C. B. Huffaker, and M. van de Vrie. “Ecology of Tetranychid Mites and Their Natural Enemies: A Review. I. Tetranychid Enemies: Their Biological Characters and the Impact of Spray Practices.” Hilgardia 40 (1970): 331–90.
Thewke, S. E., and W. R. Enns. “The Spider-Mite Complex (Acarina: Tetranychoidea).” In University of Missouri Museum Contributions Monograph 1, 1969.
Vrie, M. van de, J. A. McMurtry, and C. B. Huffaker. “Ecology of Tetranychid Mites and Their Natural Enemies: A Review III. Biology, Ecology, Pest Status, and Host Plant Relations of Tetranychids.” Hilgardia 41 (1972): 343–432.
Publication date: June 4, 2024
AG-295
Other Publications in Insect and Related Pests of Vegetables
Recommendations for the use of agricultural chemicals are included in this publication as a convenience to the reader. The use of brand names and any mention or listing of commercial products or services in this publication does not imply endorsement by NC State University or N.C. A&T State University nor discrimination against similar products or services not mentioned. Individuals who use agricultural chemicals are responsible for ensuring that the intended use complies with current regulations and conforms to the product label. Be sure to obtain current information about usage regulations and examine a current product label before applying any chemical. For assistance, contact your local N.C. Cooperative Extension county center.
N.C. Cooperative Extension prohibits discrimination and harassment regardless of age, color, disability, family and marital status, gender identity, national origin, political beliefs, race, religion, sex (including pregnancy), sexual orientation and veteran status.